Abstract
We developed a double-staining procedure involving NanoOrange dye (Molecular Probes, Eugene, Oreg.) and membrane integrity stains (LIVE/DEAD BacLight kit; Molecular Probes) to show the morphological and membrane integrity changes of Campylobacter coli cells during growth. The conversion from a spiral to a coccoid morphology via intermediary forms and the membrane integrity changes of the C. coli cells can be detected with the double-staining procedure. Our data indicate that young or actively growing cells are mainly spiral shaped (green-stained cells), but older cells undergo a degenerative change to coccoid forms (red-stained cells). Club-shaped transition cell forms were observed with NanoOrange stain. Chlorinated drinking water affected the viability but not the morphology of C. coli cells.
Campylobacter cells are mostly slender, spirally curved rods (16). When Campylobacter jejuni is exposed to suboptimal conditions, its characteristic curved, spiral morphology undergoes a transition via numerous intermediary forms until the cells finally adopt a coccoid morphology (3, 12). Cells in old cultures may form coccoid bodies, which are considered degenerative forms rather than a dormant stage of the organism (6). From the results of hundreds of studies published since 1982, it is now more clearly understood that a lack of nutrients, low temperatures, high pressure, sharp changes in pH or salinity, and other environmental factors can initiate a complex series, or cascade, of cellular events that include changes in cellular morphology and in concentration and/or structure of major biopolymers (proteins, membrane lipids, and nucleic acids) and cessation of ability to grow on solid or liquid laboratory media that would otherwise support growth of the bacterial strain employed in the studies (2).
Recently, new nucleic acid-specific dyes with different quantum yields on DNA and RNA have been developed (10). The LIVE/DEAD BacLight kit (catalog no. L-7012; Molecular Probes, Eugene, Oreg.) stain mixture (BL) distinguishes viable bacterial cells from dead ones on the basis of membrane integrity. The kit contains two nucleic acid stains. The green fluorochrome (Syto 9) is a small molecule that can penetrate intact plasma membranes, while the larger red fluorochrome (propidium iodide) penetrates only compromised membranes. Bacterial suspensions incubated in the presence of both stains simultaneously will fluoresce either green (i.e., viable) or red (i.e., dead), depending on their viability. The excitation and emission maxima for these dyes are about 480 and 500 nm for Syto 9 and 490 and 635 nm for propidium iodide, respectively (Handbook of fluorescent probes and research chemicals, Molecular Probes).
The fluorescent protein stain NanoOrange (catalog no. N-6666; Molecular Probes) has been used recently for visualizing bacterial flagella (4). The NanoOrange reagent is virtually nonfluorescent in aqueous solutions, but upon interaction with hydrophobic regions of proteins, it undergoes a dramatic fluorescence enhancement, with excitation and emission peaks averaging about 485 and 590 nm. Nucleic acids do not interfere with protein quantitation when NanoOrange reagent is used (Handbook of fluorescent probes and research chemicals, Molecular Probes).
In this paper we describe a simple and rapid method for facilitating the characterization of changes in viability and morphology during the growth of Campylobacter coli cells. This method enables visualization of cell shapes and membrane integrity changes of C. coli cells with the fluorescent membrane integrity dyes Syto 9 and propidium iodide and the fluorescent protein stain NanoOrange.
Culture conditions.
C. coli strain NCTC 11366 was employed during these studies. To monitor morphological and membrane integrity changes, the organism was grown under a microaerobic atmosphere (10% CO2, 5% O2, 85% N2) for 24 h at 37°C on a Campylobacter agar base (Oxoid) supplemented with 5% lysed sheep blood (Oxoid) (NA2). Two culture media were inoculated to produce exponential- or stationary-phase cells. A 24-h plate culture was suspended in a 100-ml Erlenmeyer flask of nutrient broth no. 2 (Oxoid) supplemented with 5% sheep blood (NB2) and was incubated at 37°C under a microaerobic atmosphere. Additionally, a 24-h plate culture was plated onto a Campylobacter agar base supplemented with 5% lysed sheep blood and incubated at 37°C under a microaerobic atmosphere. Cultures of broth and plate media were sampled at 6, 12, 20, 24, 48, and 72 h postinoculation. The morphological and membrane integrity changes were studied by the BL-NanoOrange staining method described below. All the experiments were carried out in duplicate.
Chlorine inactivation.
An inactivation experiment was conducted as follows: samples taken at different interval times (20, 24, 48, and 72 h) from the broth and plate media described above were washed twice with phosphate-buffered saline and suspended in 250 μl of filter-sterilized chlorinated drinking water (0.81 mg of total chlorine/liter and 0.75 mg of free chlorine/liter). Chlorine levels were measured with an amperometric titrator (model 716 DMS Titrino; Methrom, Herisau, Switzerland), and the physicochemical parameters of drinking water (Table 1) were determined according to American Public Health Association standards (1). Drinking water-inoculated samples were incubated for 15 min at room temperature and examined by the BL-NanoOrange staining method.
TABLE 1.
Physicochemical characteristics of tap watera
| Parameter | Amt (mg/liter) |
|---|---|
| Calcium | 120 |
| Nitrate | 9.1 |
| Phosphate | NDb |
| Silica | 2 |
| SiO2 | 4 |
| Sodium | 58 |
| Sulfate | 300 |
| Magnesium | 42 |
| Ammonia | ND |
| Total chlorine | 0.81 |
pH 7.9.
ND, not detected.
BL-NanoOrange staining method.
Samples (1 ml) were mixed with 3 μl of a mixture of Syto 9 and propidium iodide (1:1), nucleic acid stains from a LIVE/DEAD BacLight viability kit, and were incubated in the dark for 15 min at room temperature. C. coli cells stained with the BL mixture were immobilized on glass surfaces. BL-stained bacteria (5 μl) were applied to the poly-l-lysine area at the center of a clean glass microscope slide. A 0.25-μl portion of undiluted NanoOrange stock solution was added to the 5 μl of immobilized BL-stained bacteria on the microscope slide. An 18-mm2 coverslip was placed over the suspension and sealed with petrolatum. Grossart et al. (4) reported that a 1:20 dilution also worked well for staining bacterial flagella. These authors (4) observed that staining times of 10 to 15 min in the dark ensured adequate staining of bacterial flagella, whereas shorter staining times resulted in images that were qualitatively less bright and prolonged staining times (up to 6 h) did not change the quality of the image. We examined the slide after a 10-min staining period at a magnification of ×1,250 with an epifluorescence microscope (Labophot; Nikon, Tokyo, Japan) equipped with a mercury lamp and a Nikon B2-A filter set for NanoOrange stains and an Omega (Brattleboro, Vt.) XF-53 filter set for BL stains. Ideally, healthy (live) bacteria with intact plasma membranes fluoresce green and the dead or injured cells with compromised membranes fluoresce red. In accordance with the manufacturer's instructions (Handbook of fluorescent probes and research chemicals, Molecular Probes), all green cells were considered viable and red cells were considered dead. Images were recorded with a digital camera (model DP-10; Olympus, Hamburg, Germany).
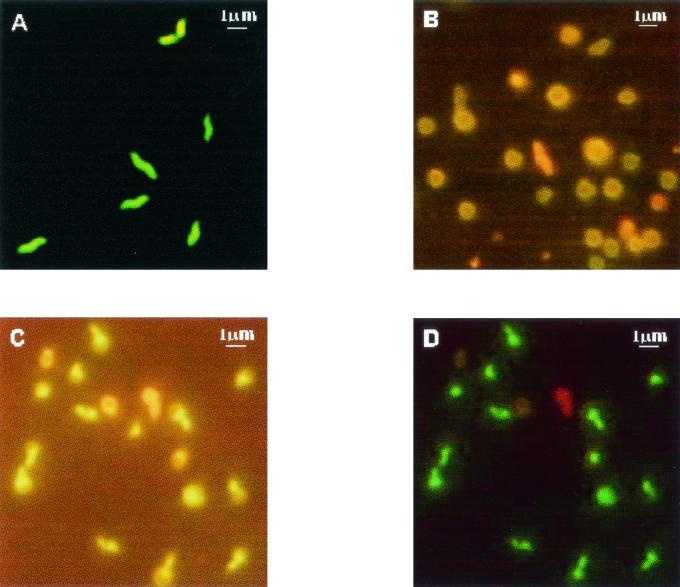
All cells observed in samples examined after 6 and 12 h were spiral shaped. Changes in cell morphology from spiral to coccoid forms and in membrane integrity were detected after BL and NanoOrange staining, as shown in Fig. 1. After 20 h, C. coli cells grown on NA2 were in spiral form and had maintained membrane integrity (Fig. 1A). Similar results were observed with C. coli cells grown on NB2. After 24 h, C. coli cells grown on NA2 showed a transition form (Fig. 1B), but the C. coli cells grown on NB2 still presented the spiral form. Ng et al. (12) observed that more than 99% of C. coli cells grown in Bacto Brucella Broth and incubated at 37°C in a modified atmosphere (7% CO2) for 24 h were spiral shaped. All the transition forms detected were club shaped. These transition forms of Campylobacter species have been demonstrated previously by using transmission electron microscopy (9, 12) and scanning electron microscopy (12, 15). The transition forms (Fig. 1C) were sometimes viable (with intact membranes) (Fig. 1D) and sometimes dead (with injured membranes) (Fig. 1D). Lázaro et al. (9) indicated that two forms of nonculturable C. jejuni cells may exist—viable and nonviable—which may not correspond with spiral and coccoid forms, respectively. Several authors (7, 13) have reported a transition to the viable but nonculturable (VBNC) stage and have found a conversion to coccoid forms, some with a slightly enlarged periplasmic space or with membrane budding. Lázaro et al. (9) observed bleb-like membrane vesicles around C. jejuni cells incubated at 4°C. These vesicles could be due to a process of cell volume adjustment by bleb formation, part of a survival strategy for minimizing cell maintenance requirements and enhancing substrate uptake with a high surface-to-volume ratio (9). Thomas et al. (15) found that the typical spiral form of C. jejuni, evident during logarithmic phase, undergoes elongation during stationary phase before becoming coccoid via the formation of membrane blebs and budded forms in decline phase. Cellular elongation and coccoid formation occurred despite the inhibition of protein synthesis and without a detectable change in the protein components of the inner and outer cell membranes (15). Jacob et al. (7) found no changes in whole-cell protein or lipooligosaccharide patterns as the cells became nonculturable. These authors (7) concluded that coccoid forms may be considered dormant forms that would not be detected in water by conventional microbiological methods. Tholozan et al. (14) recorded an increase in cell volume of VBNC C. jejuni cells, which had significantly lower potassium contents and membrane potentials than cells in the culturable stage. All these traits were suggested to be involved in strategies for minimizing cell maintenance requirements. The involvement of membrane blebs and budding in the process of coccoid formation in the absence of de novo protein synthesis and without changes in membrane protein composition indicates that the process is passive and potentially degenerative (15). There is evidence that the formation of coccoid C. jejuni cells is not an active process but represents a degeneration resulting from oxidative damage (5). After 72 h, C. coli cells showed coccoid forms. It has been proposed that even though these coccoid forms are not culturable, they may still be viable (13). However, the evidence that the coccoid forms can revert back to the spiral forms is generally weak, and there is considerable controversy surrounding the whole phenomenon of VBNC cells (8).
FIG. 1.
Double staining of C. coli cells demonstrates the morphology and membrane integrity changes during growth in broth and plate media. (A) Viable (green) spiral form cells of a 20-h culture on NB2, stained with Syto 9, visualized with an XF-53 filter; (B) transition and coccoid form cells of a 48-h culture on NA2, stained with NanoOrange, visualized with a B2-A filter; (C) club-shaped cells of a 24-h culture on NA2, stained with NanoOrange, visualized with a B2-A filter; (D) viable (green) and dead (red) club-shaped cells of a 24-h culture on NA2, stained with the BL stain mixture, visualized with an XF-53 filter.
All Campylobacter cells (spiral, transition, and coccoid forms) taken at different time intervals (20, 24, 48, and 72 h) from broth and plate media and suspended in 250 μl of filter-sterilized chlorinated drinking water for 15 min showed injured membranes (they stained red). Chlorinated drinking water affected the viability but not the morphology of C. coli cells. The same cell shape observed on NA2 or NB2 was detected after cell suspension in chlorinated drinking water. Only membrane integrity was compromised. Exposure to chlorine has been shown to damage cell membranes (11).
In summary, we describe a novel staining method for rapid visualization of morphological and membrane integrity changes of C. coli cells. Like the NanoOrange method for demonstrating bacterial flagella (4), the BL-NanoOrange method does not require fixation of the cells and involves very little sample manipulation; thus, artifacts due to fixation, dehydration, or excessive sample manipulation can be avoided.
Acknowledgments
This work was supported by grant no. ALI99-0539 from CICYT. S.M. has a graduate student fellowship from the Universidad Politécnica de Valencia.
We thank Mariano Ferris for physicochemical characterization of drinking water and Richard Connon for correcting the English in the manuscript.
REFERENCES
- 1.American Public Health Association. 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, D.C.
- 2.Colwell, R. R., and D. J. Grimes. 2000. Semantics and strategies, p. 1-6. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 3.Griffiths, P. L. 1993. Morphological changes of Campylobacter jejuni growing in liquid culture. Lett. Appl. Microbiol. 17:152-155. [DOI] [PubMed] [Google Scholar]
- 4.Grossart, H.-P., G. F. Steward, J. Martinez, and F. Azam. 2000. A simple, rapid method for demonstrating bacterial flagella. Appl. Environ. Microbiol. 66:3632-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey, P., and S. Leach. 1998. Analysis of coccal cell formation by Campylobacter jejuni using continuous culture techniques, and the importance of oxidative stress. J. Appl. Microbiol. 85:398-404. [DOI] [PubMed] [Google Scholar]
- 6.Hazeleger, W., C. Arkesteijn, A. Toorop-Bouma, and R. Beumer. 1994. Detection of the coccoid form of Campylobacter jejuni in chicken products with the use of the polymerase chain reaction. Int. J. Food Microbiol. 24:273-281. [DOI] [PubMed] [Google Scholar]
- 7.Jacob, J., W. Martin, and C. Holler. 1993. Characterization of viable but nonculturable stage of C. coli, characterized with respect to electron microscopic findings, whole cell protein and lipooligosaccharide (LOS) patterns. Zentbl. Mikrobiol. 148:3-10. (In German.) [PubMed] [Google Scholar]
- 8.Kelly, D. 2001. The physiology and metabolism of Campylobacter jejuni and Helicobacter pylori. J. Appl. Microbiol. 90:16-24. [DOI] [PubMed] [Google Scholar]
- 9.Lázaro, B., J. Cárcamo, A. Audícana, I. Perales, and A. Fernández-Astorga. 1999. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 65:4677-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebaron, P., N. Parthuisot, and P. Catala. 1998. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl. Environ. Microbiol. 64:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisle, J. T., S. C. Broadaway, A. M. Prescott, B. H. Pyle, C. Fricker, and G. A. McFeters. 1998. Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli 0157:H7. Appl. Environ. Microbiol. 64:4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng, L.-K., R. Sherburne, D. E. Taylor, and M. E. Stiles. 1985. Morphological forms and viability of Campylobacter species studied by electron microscopy. J. Bacteriol. 164:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tholozan, J. L., J. M. Cappelier, J. P. Tissier, G. Delattre, and M. Federighi. 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas, C., D. J. Hill, and M. Mabey. 1999. Morphological changes of synchronized Campylobacter jejuni populations during growth in single phase liquid culture. Lett. Appl. Microbiol. 28:194-198. [DOI] [PubMed] [Google Scholar]
- 16.Vandamme, P. 2000. Taxonomy of the family Campylobacteraceae, p. 3-26. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.