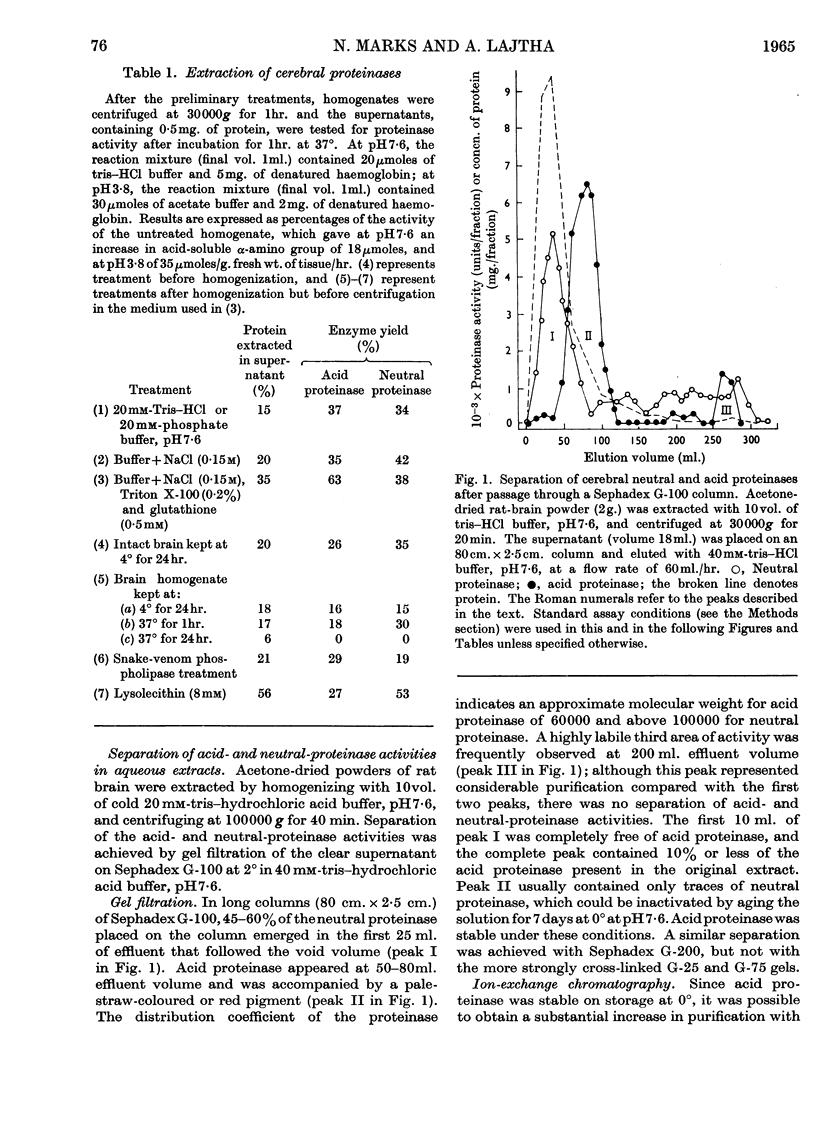
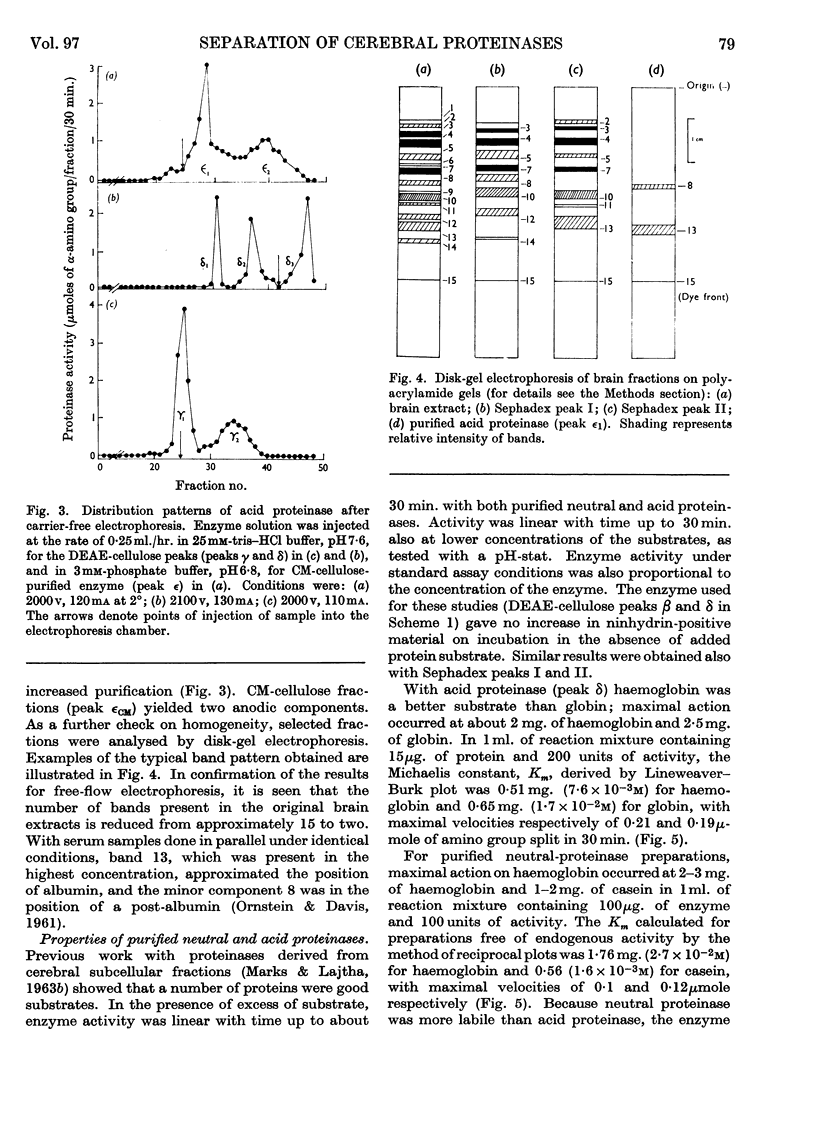
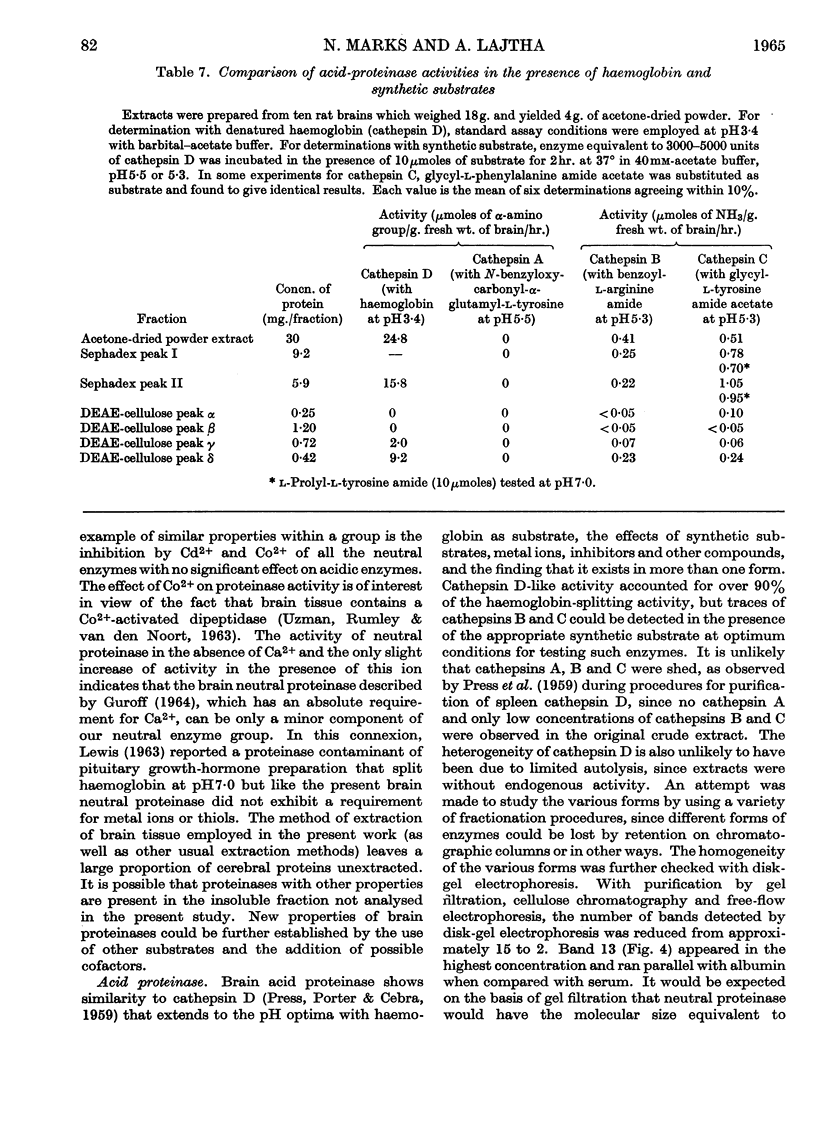
Abstract
1. Cerebral proteinases were separated on Sephadex G-100 columns into acid and neutral fractions free from cross-contamination. Acid proteinases were more stable and were purified by additional steps with salt and pH5·0 precipitations, column chromatography on DEAE- or CM-cellulose and free-flow electrophoresis. 2. The separation made it possible to study the properties of the partially purified enzyme fractions. Some of these properties, such as Km with selected protein substrates, pH optima and temperature-dependence in the presence and absence of substrates, are described. 3. No requirement for metal ions or added cofactors was demonstrated. Neutral-proteinase activity was more sensitive to inhibition by heavy-metal ions; its activity could be increased by thioglycollate and glutathione, and inhibited by thiol reagents. Neutral and acid proteinases were inhibited by the chymotrypsin inhibitor chloromethyl l-2-phenyl-1-toluene-p-sulphonamidoethyl ketone. 4. In the presence of the appropriate synthetic substrates no cathepsin A activity was found, and only trace quantities of cathepsin B or C activities, which were more than 50-fold less than cathepsin D-like activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANSELL G. B., RICHTER D. Evidence for a neutral proteinase in brain tissue. Biochim Biophys Acta. 1954 Jan;13(1):92–97. doi: 10.1016/0006-3002(54)90276-0. [DOI] [PubMed] [Google Scholar]
- ANSELL G. B., RICHTER D. The proteolytic activity of brain tissue. Biochim Biophys Acta. 1954 Jan;13(1):87–91. doi: 10.1016/0006-3002(54)90275-9. [DOI] [PubMed] [Google Scholar]
- BAUER H., MATZELT D., SCHWARZE I. [Research on brain proteins during the action of lysolecithin on brain homogenates]. Klin Wochenschr. 1962 Mar 1;40:251–255. doi: 10.1007/BF01477107. [DOI] [PubMed] [Google Scholar]
- BOUMA J. M., GRUBER M. THE DISTRIBUTION OF CATHEPSINS B AND C IN RAT TISSUES. Biochim Biophys Acta. 1964 Sep 18;89:545–547. doi: 10.1016/0926-6569(64)90082-3. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., SHERMAN R. Studies on catheptic carboxypeptidase. J Biol Chem. 1962 Apr;237:1082–1085. [PubMed] [Google Scholar]
- GUROFF G. A NEUTRAL, CALCIUM-ACTIVATED PROTEINASE FROM THE SOLUBLE FRACTION OF RAT BRAIN. J Biol Chem. 1964 Jan;239:149–155. [PubMed] [Google Scholar]
- IMAI Y., SATO R. Solubilization of aromatic hydroxylase system of liver microsomes and requirement of lipid-like factor. Biochim Biophys Acta. 1960 Jul 29;42:164–165. doi: 10.1016/0006-3002(60)90767-8. [DOI] [PubMed] [Google Scholar]
- LAPRESLE C., WEBB T. The purification and properties of a proteolytic enzyme, rabbit cathepsin E, and further studies on rabbit cathepsin D. Biochem J. 1962 Sep;84:455–462. doi: 10.1042/bj0840455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS U. J. FACTORS THAT AFFECT THE FRAGMENTATION OF GROWTH HORMONE AND PROLACTIN BY HYPOPHYSIAL PROTEINASES. J Biol Chem. 1963 Oct;238:3330–3335. [PubMed] [Google Scholar]
- LISHKO V. K. VYVCHENNIA VLASTYVOSTE I KATEPSYNU MOZKU. Ukr Biokhim Zh. 1963;35:874–880. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKS N., LAJTHA A. PROTEIN BREAKDOWN IN THE BRAIN. SUBCELLULAR DISTRIBUTION AND PROPERTIES OF NEUTRAL AND ACID PROTEINASES. Biochem J. 1963 Dec;89:438–447. doi: 10.1042/bj0890438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBRINK K. J. A modified Conway unit for microdiffusion analysis. Biochem J. 1955 Jan;59(1):134–136. doi: 10.1042/bj0590134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON E. A., CHIAZZE E. A. Some experimental factors in the gradient chromatography of serum proteins. Arch Biochem Biophys. 1962 Oct;99:136–147. doi: 10.1016/0003-9861(62)90256-4. [DOI] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- UZMAN L. L., RUMLEY M. K., VANDENNOORT S. THE INHIBITION OF CEREBRAL DIGLYCINASE BY ALPHA-AMINO-, ALPHA-KETO- AND ALPHA-HYDROXY ACIDS. J Neurochem. 1963 Dec;10:795–804. doi: 10.1111/j.1471-4159.1963.tb11904.x. [DOI] [PubMed] [Google Scholar]


