Abstract
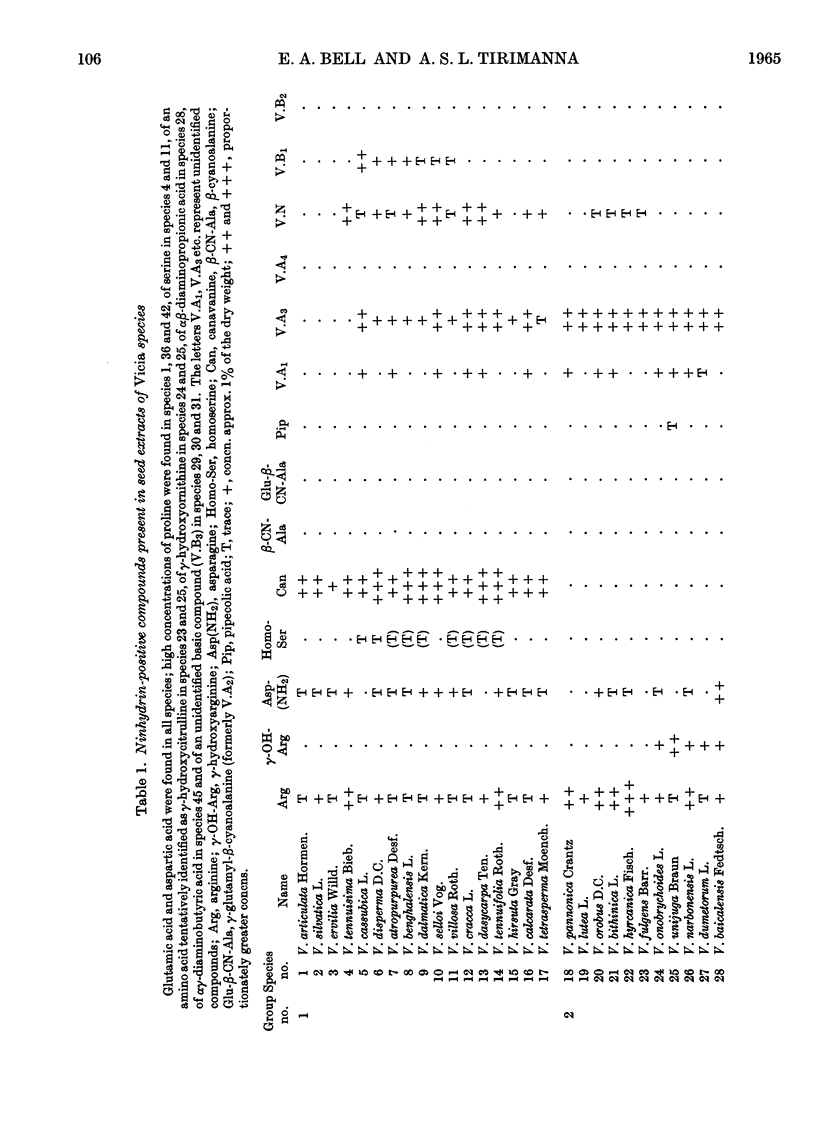
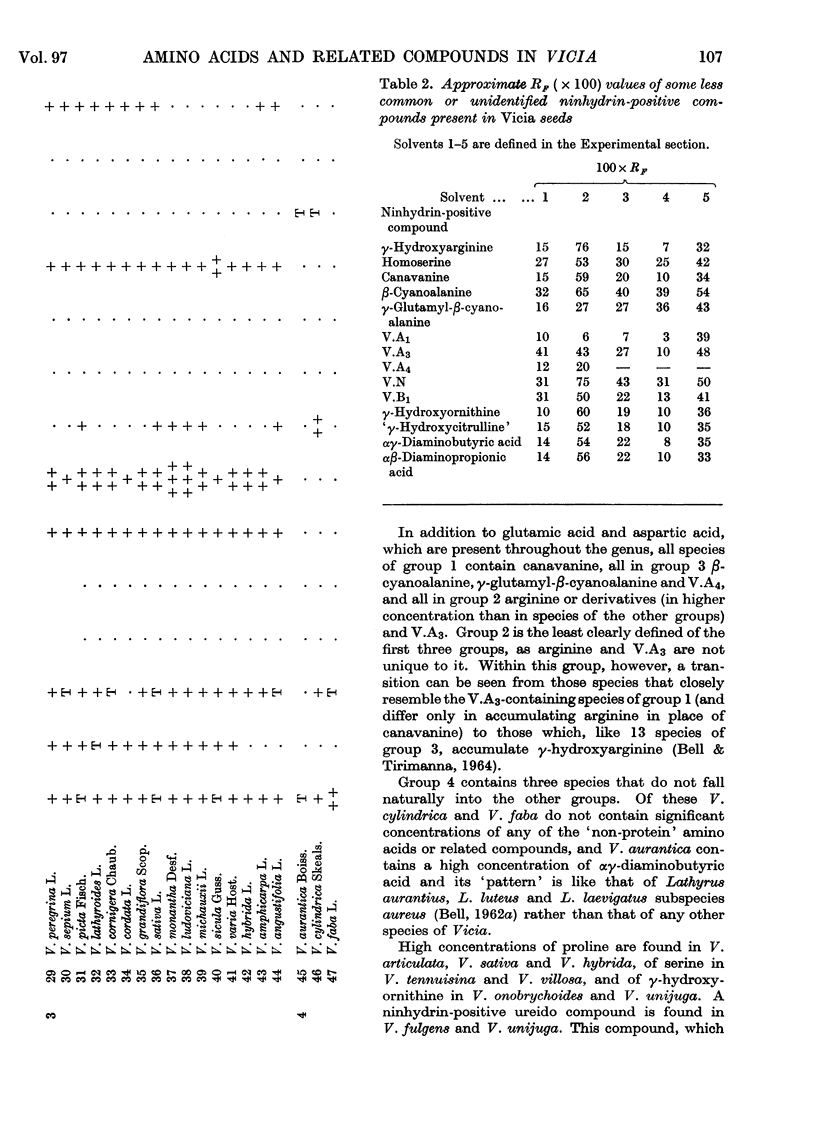
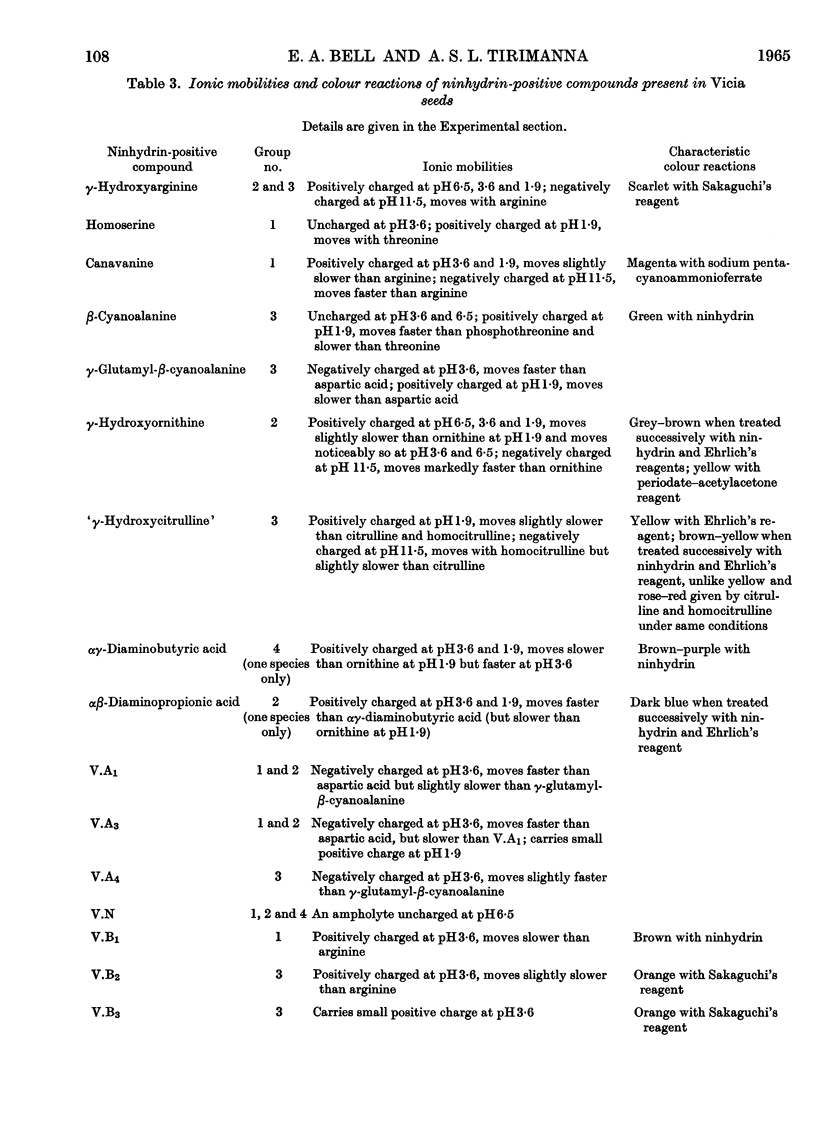
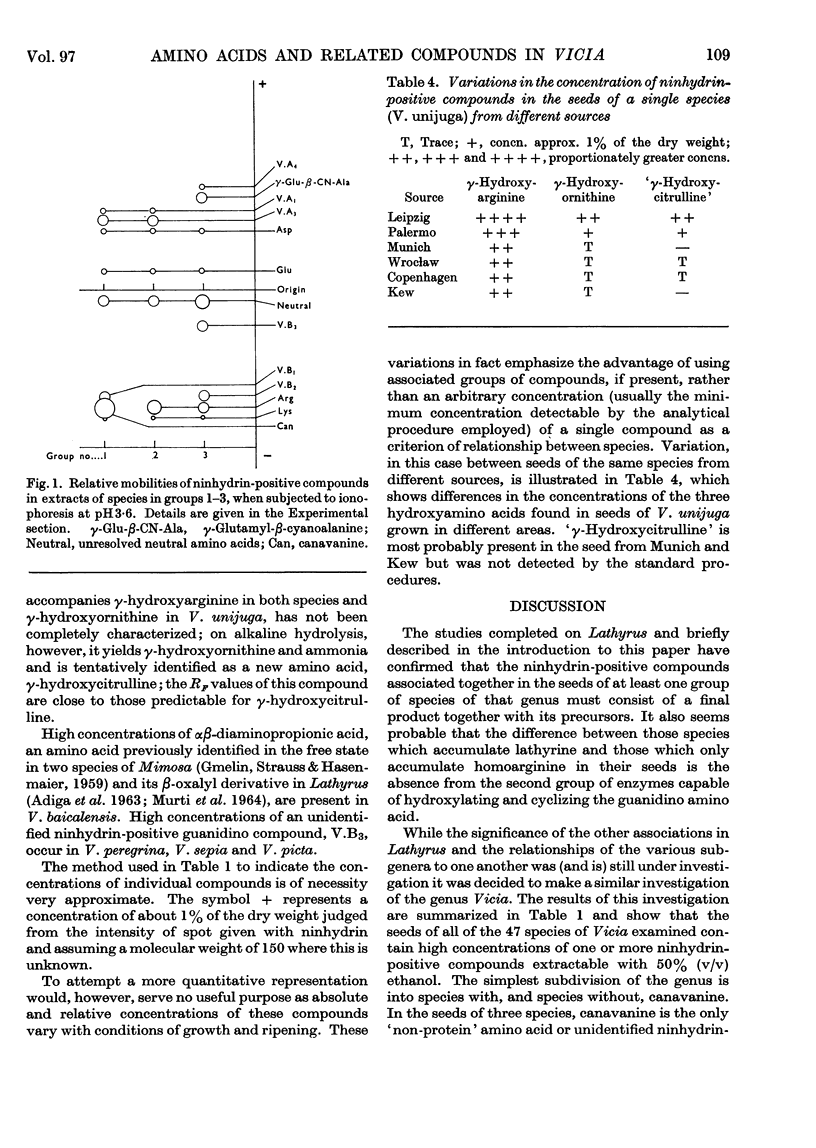
1. The genus Vicia may be subdivided into groups of species characterized by associations of ninhydrin-positive compounds occurring in high concentrations in their seeds. Despite these subdivisions the overall distribution pattern emphasizes the unity of the genus and the possible value of such studies in establishing degrees of relationship between species within the genus. 2. Canavanine is a major constituent in the seeds of 17 species. 3. γ-Hydroxyarginine, an amino acid that has not been found in other plant genera, occurs in 18 species. 4. γ-Hydroxyornithine, a new natural amino acid, is found in two species. 5. A new naturally occurring ureido amino acid tentatively identified as `γ-hydroxycitrulline' is found in two species. 6. High concentrations of αβ-diaminopropionic acid are found in seed of V. baicalensis and of αγ-diaminobutyric acid in seed of V. aurantica. 7. The neurotoxic amino acid β-cyanoalanine and its γ-glutamyl peptide are found together in high concentrations in the seeds of 16 species of this agriculturally important genus. 8. Seven unidentified ninhydrin-positive compounds occur in high concentration (about 1% of the dry weight or more) in the seed of various species. Details of their RF values, ionic mobilities and colour reactions are given. 9. The total concentration of extractable ninhydrin-positive compounds varies little between seeds of different species and these compounds may, as has been suggested for those in Lathyrus, constitute a readily available form of storage nitrogen. 10. The nature and distribution, as opposed to the total concentration, of the amino acids and related compounds accumulated in the seeds of Vicia are different from those accumulated in the seeds of the related genus, Lathyrus. One particularly interesting difference is the accumulation of C6 guanidino amino acids (arginine and γ-hydroxyarginine) by Vicia and the accumulation of C7 guanidino amino acids (homoarginine, γ-hydroxyhomoarginine and the related amino acid lathyrine) by Lathyrus. Such differences afford a method for distinguishing between species of these genera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. A. Associations of ninhydrin-reacting compounds in the seeds of 49 species of Lathyrus. Biochem J. 1962 May;83:225–229. doi: 10.1042/bj0830225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. A. Canavanine and related compounds in Leguminosae. Biochem J. 1958 Dec;70(4):617–619. doi: 10.1042/bj0700617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. A., FOSTER R. G. Structure of lathyrine. Nature. 1962 Apr 7;194:91–92. doi: 10.1038/194091a0. [DOI] [PubMed] [Google Scholar]
- BELL E. A. RELEVANCE OF BIOCHEMICAL TAXONOMY TO THE PROBLEM OF LATHYRISM. Nature. 1964 Jul 25;203:378–380. doi: 10.1038/203378a0. [DOI] [PubMed] [Google Scholar]
- BELL E. A. The isolation of L-homoarginine from seeds of Lathyrus cicera. Biochem J. 1962 Oct;85:91–93. doi: 10.1042/bj0850091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. A., Tirimanna A. S. The isolation of gamma-hydroxyarginine, as its lactone, from seeds of Vicia sativa, and the identification of gamma-hydroxyornithine as a naturally occurring amino acid. Biochem J. 1964 May;91(2):356–358. doi: 10.1042/bj0910356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERRITSEN T., WAISMAN H. A., LIPTON S. H., STRONG F. M. Natural occurrence of homocitrulline. I. Excretion in the urine. Arch Biochem Biophys. 1962 Apr;97:34–36. doi: 10.1016/0003-9861(62)90040-1. [DOI] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- RESSLER C. Isolation and identification from common vetch of the neurotoxin beta-cyano-L-alanine, a possible factor in neurolathyrism. J Biol Chem. 1962 Mar;237:733–735. [PubMed] [Google Scholar]
- RESSLER C., REDSTONE P. A., ERENBERG R. H. Isolation and identification of a neuroactive factor from Lathyrus latifolius. Science. 1961 Jul 21;134(3473):188–190. doi: 10.1126/science.134.3473.188. [DOI] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]


