Abstract
1. A proteolytic enzyme with some features of a carboxypeptidase has been purified some 1180-fold from the sap of French beans (Phaseolus vulgaris var. Prince). A bright blue protein, plastocyanin, was separated from the enzyme by DEAE-cellulose chromatography. 2. Unlike carboxypeptidase A or B of animal origin, there is no evidence that the enzyme is a metalloprotein. There was no stimulation of activity by a number of metal ions, reducing agents or 2-mercapto-ethanol. Neither EDTA nor 1,10-o-phenanthroline inhibited the enzyme. 3. The proteolytic enzyme from beans, readily soluble at neutral or slightly acidic pH values, has a pH optimum of pH5·6 for the hydrolysis of leucine from benzyloxy-carbonylglycyl-l-leucine. Solutions of the enzyme in 0·1m-sodium acetate, pH5·5, lose about 2% of their activity/week at 4°. Virtually no loss of activity results after prolonged storage at −15°. 4. Incubation of the bean enzyme with peptides indicates that the enzyme will release acidic, neutral and basic amino acid residues as well as proline, although adjacent acidic residues in a peptide appear to inhibit the enzyme. The possibility of endopeptidase activity in the purified preparation requires further examination.
Full text
PDF


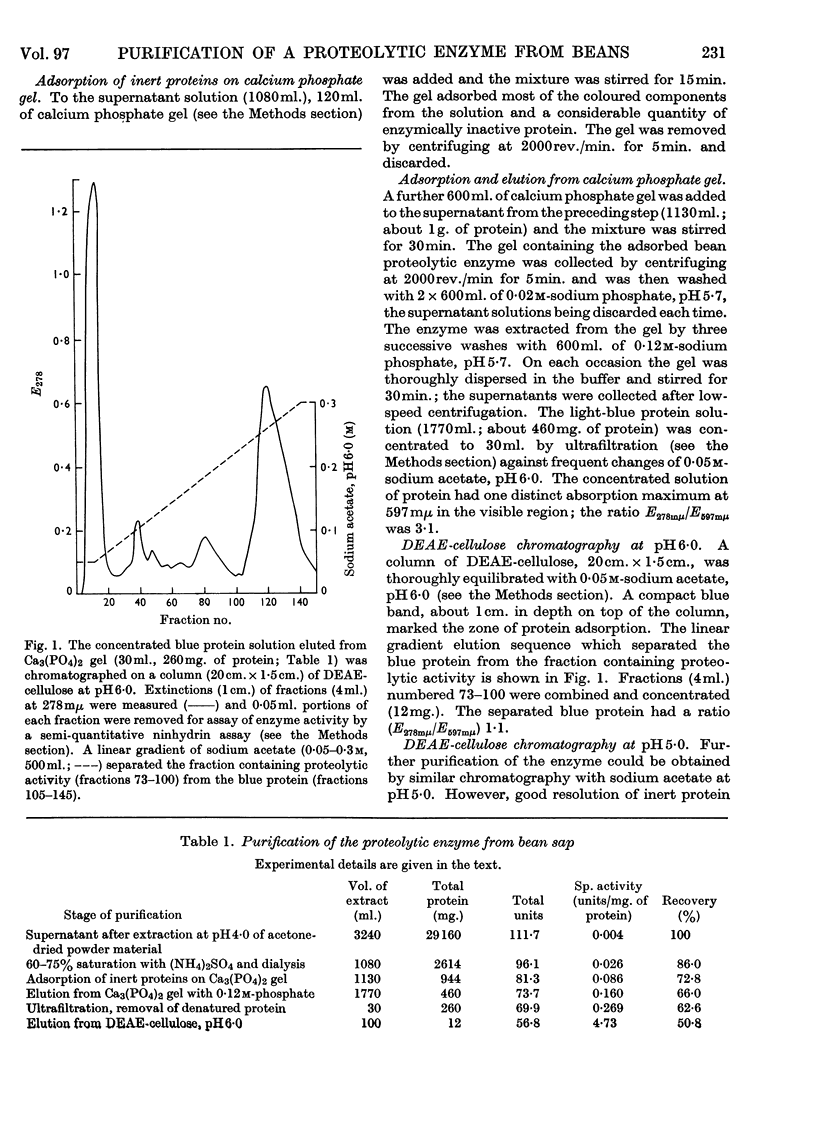
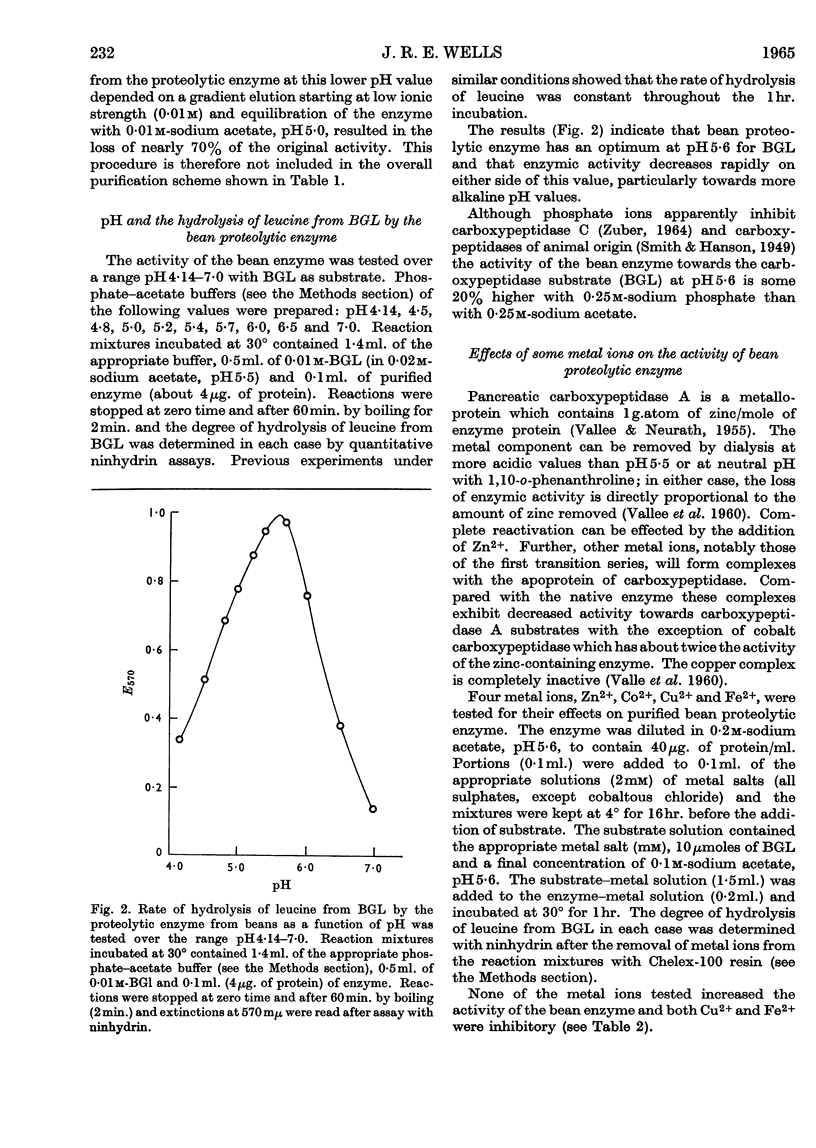



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERER F. A., UHLIG H., WEBER E., SCHRAMM G. Primary structure of the protein of tobacco mosaic virus. Nature. 1960 Jun 18;186:922–925. doi: 10.1038/186922a0. [DOI] [PubMed] [Google Scholar]
- CHIBNALL A. C., MANGAN J. L., REES M. W. Studies on the amide and C-terminal residues in proteins. 2. The ammonia nitrogen and amide nitrogen of various native protein preparations. Biochem J. 1958 Jan;68(1):111–114. doi: 10.1042/bj0680111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957 Aug;4(1):1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- GIBBINS L. N., NORRIS F. W. Phytase and acid phosphatase in the dwarf bean, Phaseolus vulgaris. Biochem J. 1963 Jan;86:67–71. doi: 10.1042/bj0860067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. I., KNIGHT C. A. Studies on the action of carboxypeptidase on tobacco mosaic virus. J Biol Chem. 1955 May;214(1):215–230. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millerd A., Morton R. K., Wells J. R. Oxalic acid synthesis in shoots of Oxalis pes-caprae. The precursors of glycollic acid and glyoxylic acid. Biochem J. 1963 Aug;88(2):276–281. doi: 10.1042/bj0880276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. D., MARKHAM R. Chromatographic studies on nucleic acids; the quantitative analysis of ribonucleic acids. Biochem J. 1950 May;46(5):509–513. doi: 10.1042/bj0460509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugita A., Gish D. T., Young J., Fraenkel-Conrat H., Knight C. A., Stanley W. M. THE COMPLETE AMINO ACID SEQUENCE OF THE PROTEIN OF TOBACCO MOSAIC VIRUS. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1463–1469. doi: 10.1073/pnas.46.11.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLEE B. L., NEURATH H. Carboxypeptidase, a zinc metalloenzyme. J Biol Chem. 1955 Nov;217(1):253–261. [PubMed] [Google Scholar]
- ZUBER H. PURIFICATION AND PROPERTIES OF A NEW CARBOXYPEPTIDASE FROM CITRUS FRUIT. Nature. 1964 Feb 8;201:613–613. doi: 10.1038/201613a0. [DOI] [PubMed] [Google Scholar]


