Abstract
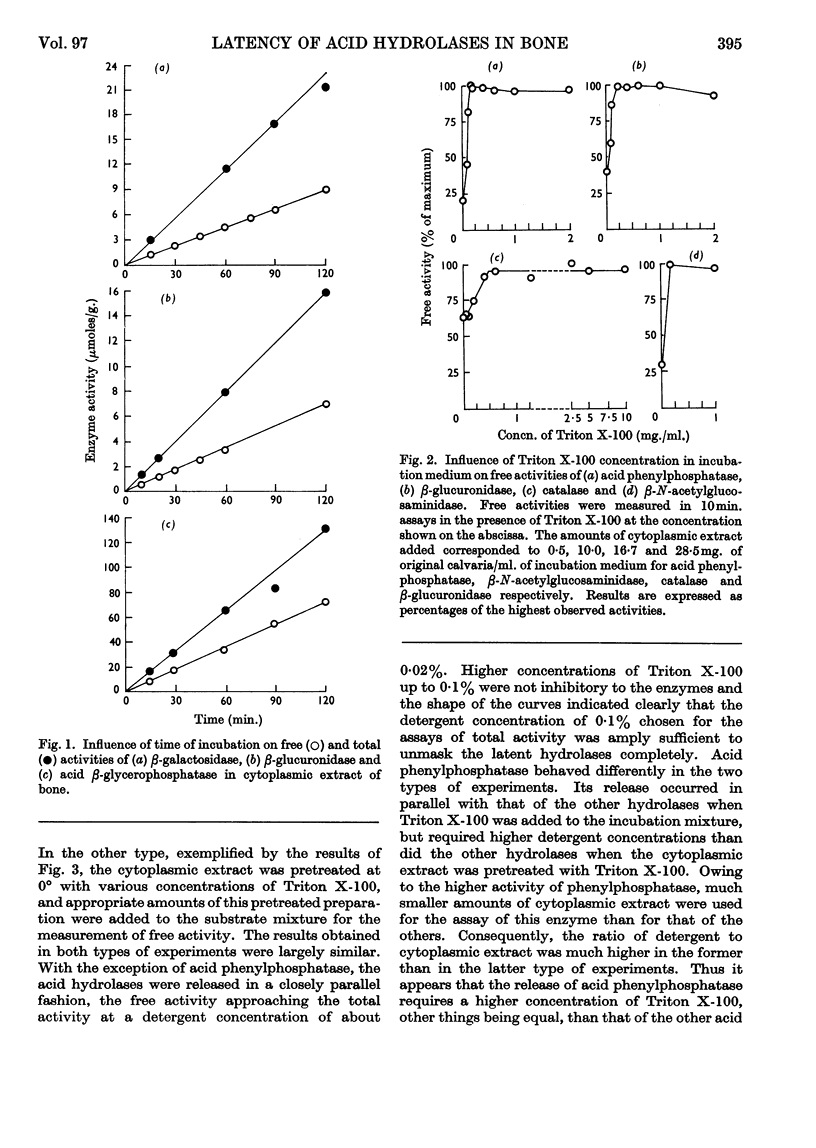
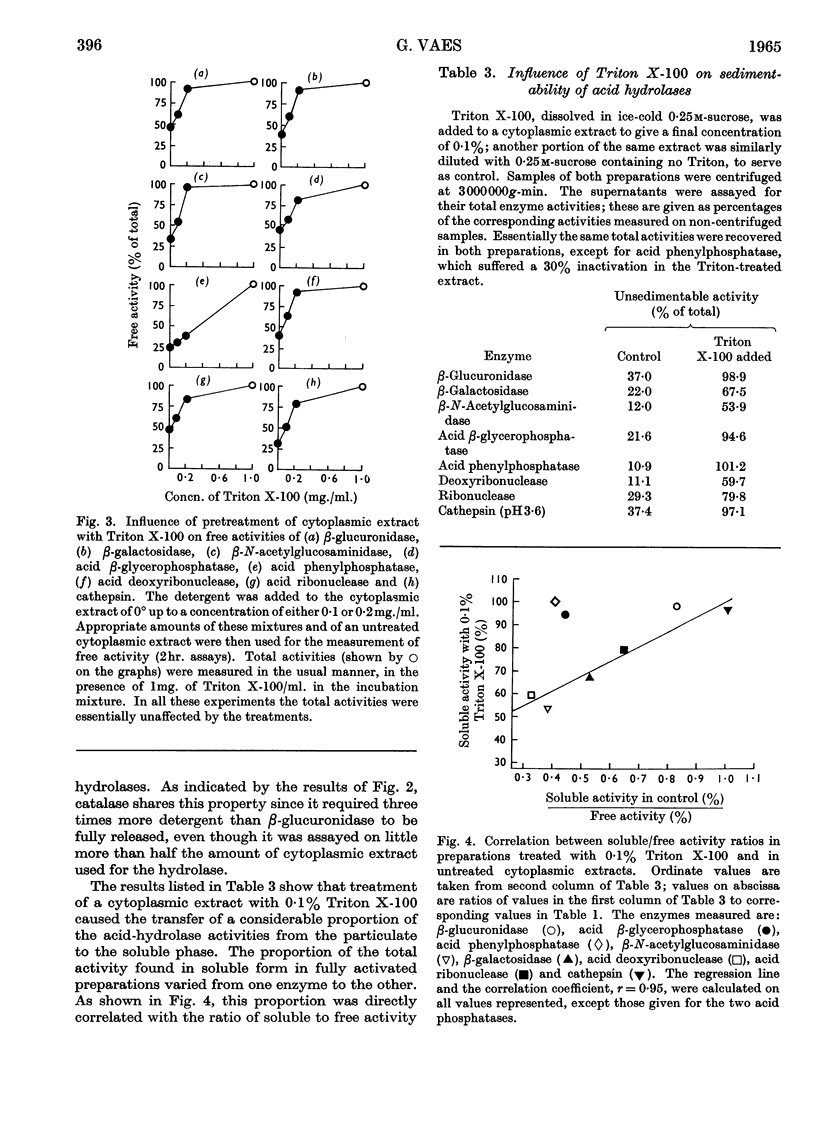
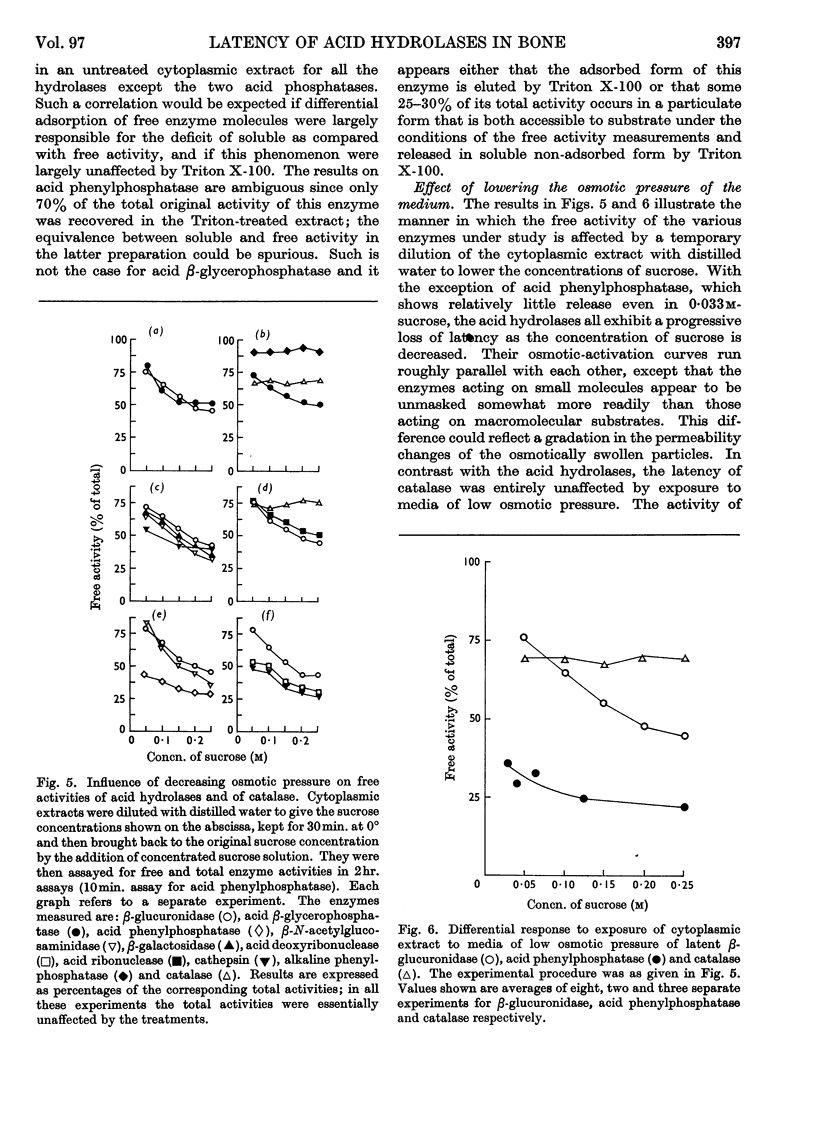
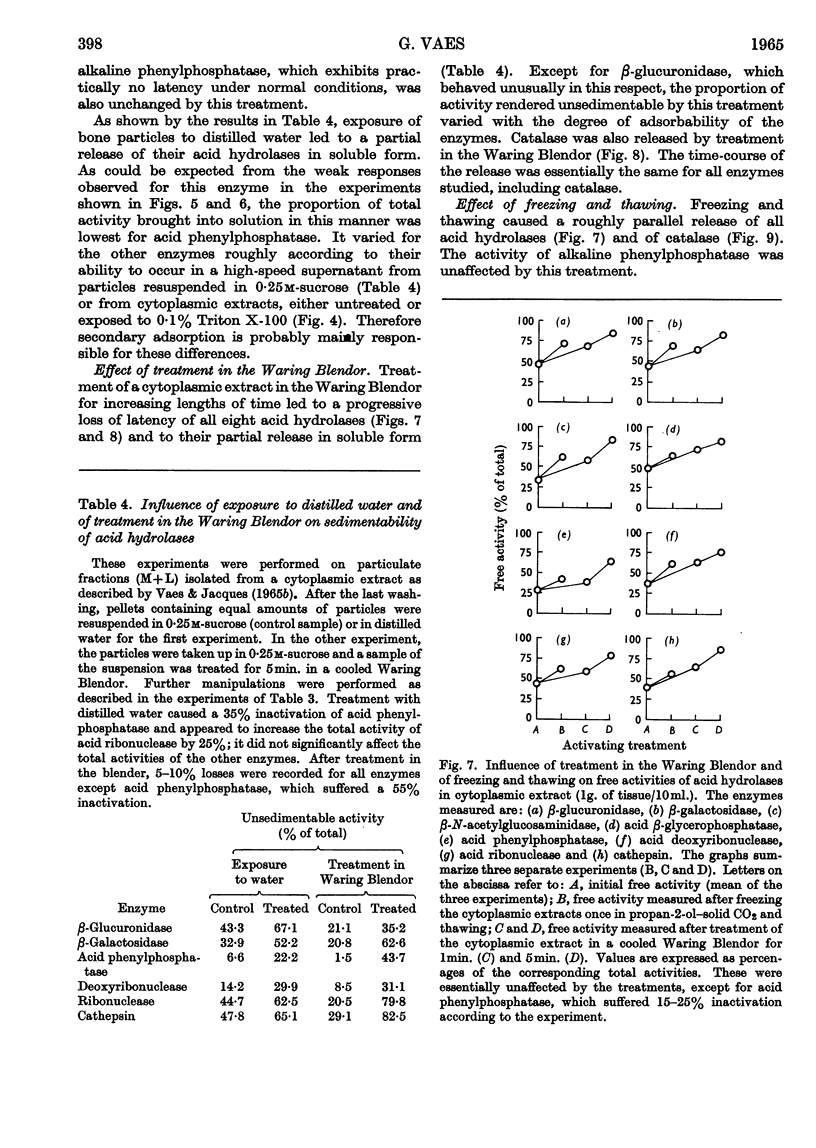
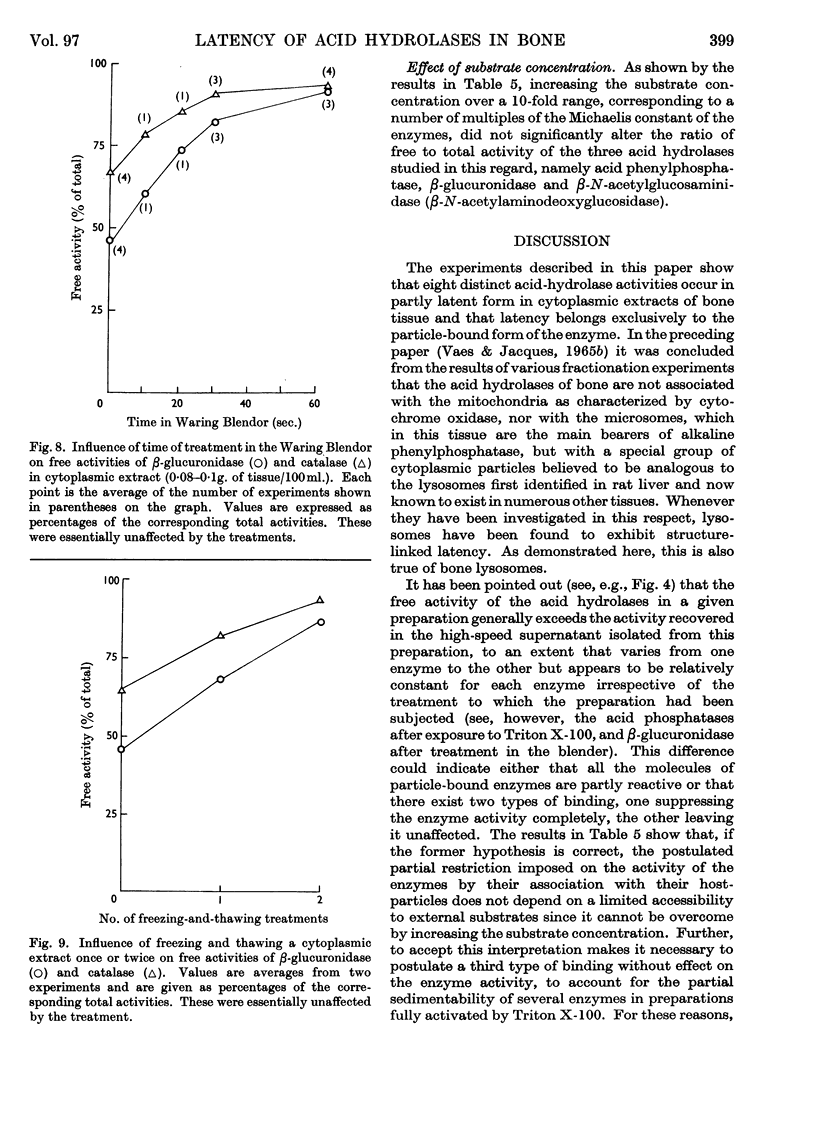
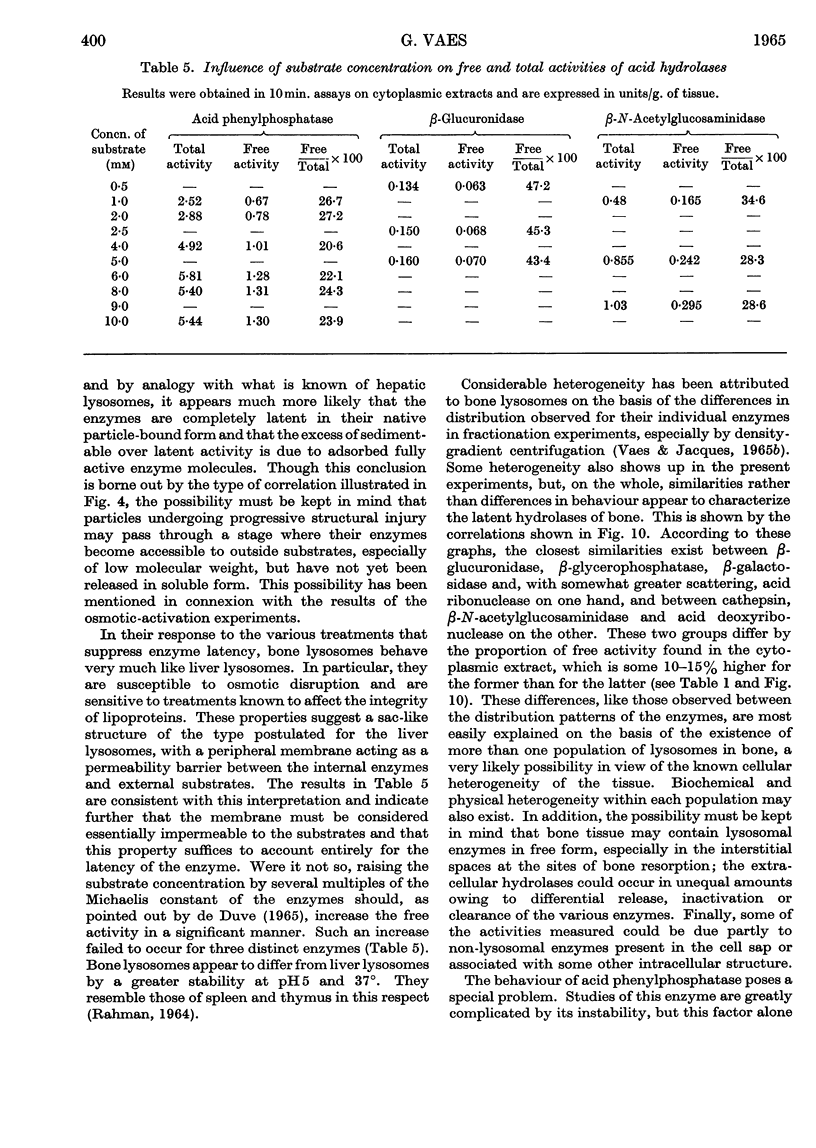
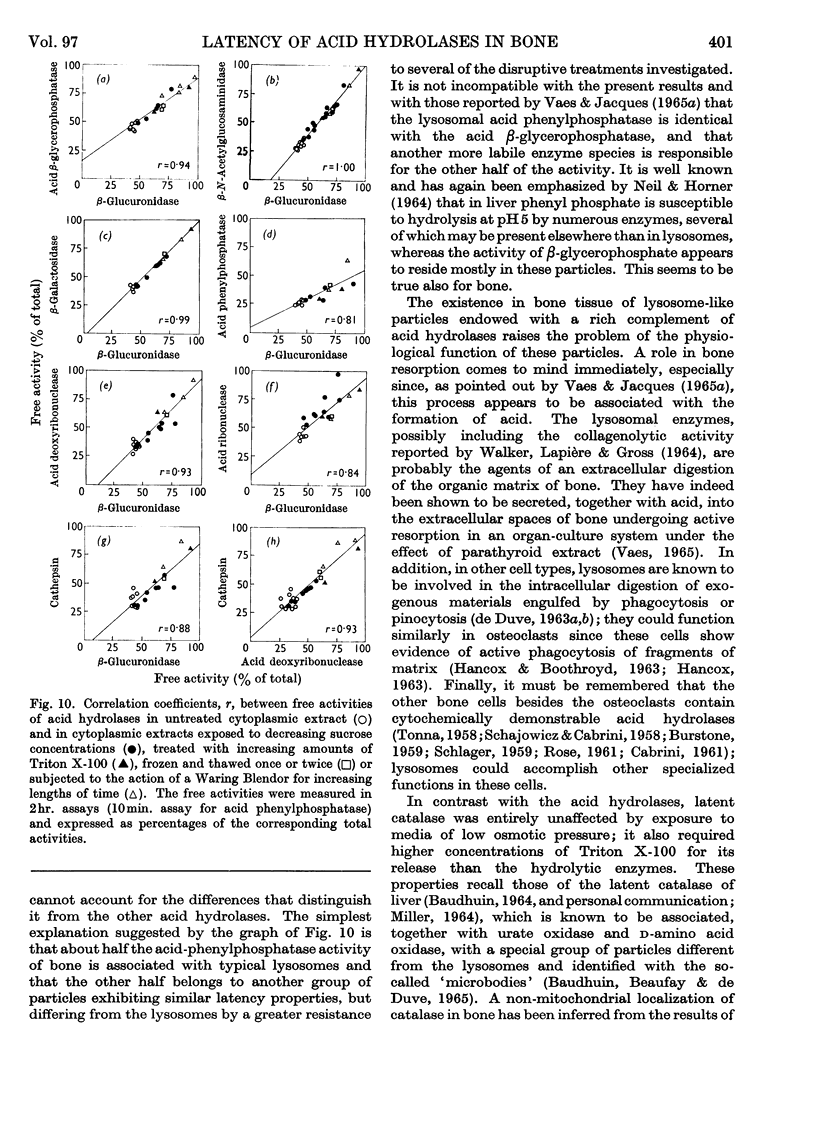
1. Eight distinct acid-hydrolase activities present in cytoplasmic extracts from bone tissue occur in latent form to the extent of 50–70% of their total activity, depending on the enzyme. 2. This latency can be decreased or suppressed by exposure to Triton X-100 or to media of low osmotic pressure, by treatment in the Waring Blendor, and by freezing and thawing, but not by increasing the substrate concentration in the assay medium up to 10-fold the Michaelis constant of the enzymes. 3. Latency is the property of the particle-bound enzymes, and treatments that suppress latency simultaneously cause solubilization of the enzymes. Most enzymes show an excess of free over soluble activity; the magnitude of this excess seems to depend largely on the nature of the enzyme, and sometimes also on the kind of treatment suffered by the preparations; it is attributed mainly to adsorption artifacts. 4. In preparations subjected to graded activating treatments, seven of the eight acid hydrolases studied are released in closely parallel fashion, suggesting that they are associated with particles possessing similar properties. Acid phenylphosphatase is released less readily than the other enzymes by Triton X-100 and by exposure to media of low osmotic pressure. 5. It is concluded from these and previous published fractionation experiments that, with the possible exception of part of the acid-phenylphosphatase activity, the eight acid hydrolases studied belong to lysosome-like particles. Bone lysosomes exhibit a relatively high degree of biochemical and physical heterogeneity. Their possible functions are discussed. Part of the acid-phenylphosphatase activity could be linked to another group of particles. 6. Catalase is also partly (30%) latent in cytoplasmic extracts of bone. Latent catalase can be released by some of the treatments that suppress the latency of the lysosomal enzymes, but differs from the latter by a greater resistance to Triton X-100, and, especially, by a complete insensitivity to exposure to media of low osmotic pressure. It is concluded from these results that the catalase-containing particles are probably different from lysosomes, as they are in liver. 7. Cytochrome oxidase, which is presumably associated with the mitochondria, and alkaline phenylphosphatase, an enzyme occurring predominantly in the microsomal fraction, exhibited no latency under the conditions of the present experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., DE DUVE C. Tissue fractionation studies. 3. Further observations on the binding of acid phosphatase by rat-liver particles. Biochem J. 1955 Mar;59(3):426–433. doi: 10.1042/bj0590426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H., JACQUES P., RAHMAN-LI Y., SELLINGER O. Z., WATTIAUX R., DE CONINCK S. Intracellular localization of catalase and of some oxidases in rat liver. Biochim Biophys Acta. 1960 May 6;40:186–187. doi: 10.1016/0006-3002(60)91338-x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R. Tissue fractionation studies. VII. Release of bound hydrolases by means of triton X-100. Biochem J. 1956 Aug;63(4):606–608. doi: 10.1042/bj0630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C. The separation and characterization of subcellular particles. Harvey Lect. 1965;59:49–87. [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil M. W., Horner M. W. Studies on acid hydrolases in adult and foetal tissues. 2. Acid phenyl phosphomonoesterases of adult mouse liver. Biochem J. 1964 Oct;93(1):220–224. doi: 10.1042/bj0930220_pt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMAN Y. E. A NOTE ON ACID PHOSPHATASE RELEASE FROM SPLEEN, LIVER AND THYMUS OF RATS. Biochim Biophys Acta. 1964 Aug 19;90:440–442. doi: 10.1016/0304-4165(64)90221-1. [DOI] [PubMed] [Google Scholar]
- ROSE G. G. The Golgi complex in living osteoblasts. J Biophys Biochem Cytol. 1961 Feb;9:463–478. doi: 10.1083/jcb.9.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAJOWICZ F., CABRINI R L. Histochemical localization of acid phosphatase in bone tissue. Science. 1958 Jun 20;127(3312):1447–1448. doi: 10.1126/science.127.3312.1447. [DOI] [PubMed] [Google Scholar]
- SCHLAGER F. [Occurrence and localization of beta-D-galactosidase in bone, cartilage and adjacent tissue of white mice]. Acta Histochem. 1959 Oct 13;8:176–184. [PubMed] [Google Scholar]
- TONNA E. A. Histologic and histochemical studies on the periosteum of male and female rats at different ages. J Gerontol. 1958 Jan;13(1):14–19. doi: 10.1093/geronj/13.1.14. [DOI] [PubMed] [Google Scholar]
- Vaes G., Jacques P. Studies on bone enzymes. Distribution of acid hydrolases, alkaline phenylphosphatase, cytochrome oxidase and catalase in subcellular fraction of bone tissue homogenates. Biochem J. 1965 Nov;97(2):389–392. doi: 10.1042/bj0970389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Jacques P. Studies on bone enzymes. The assay of acid hydrolases and other enzymes in bone tissue. Biochem J. 1965 Nov;97(2):380–388. doi: 10.1042/bj0970380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Lapiere C. M., Gross J. A collagenolytic factor in rat bone promoted by parathyroid extract. Biochem Biophys Res Commun. 1964 Apr 22;15(5):397–402. doi: 10.1016/0006-291x(64)90474-7. [DOI] [PubMed] [Google Scholar]


