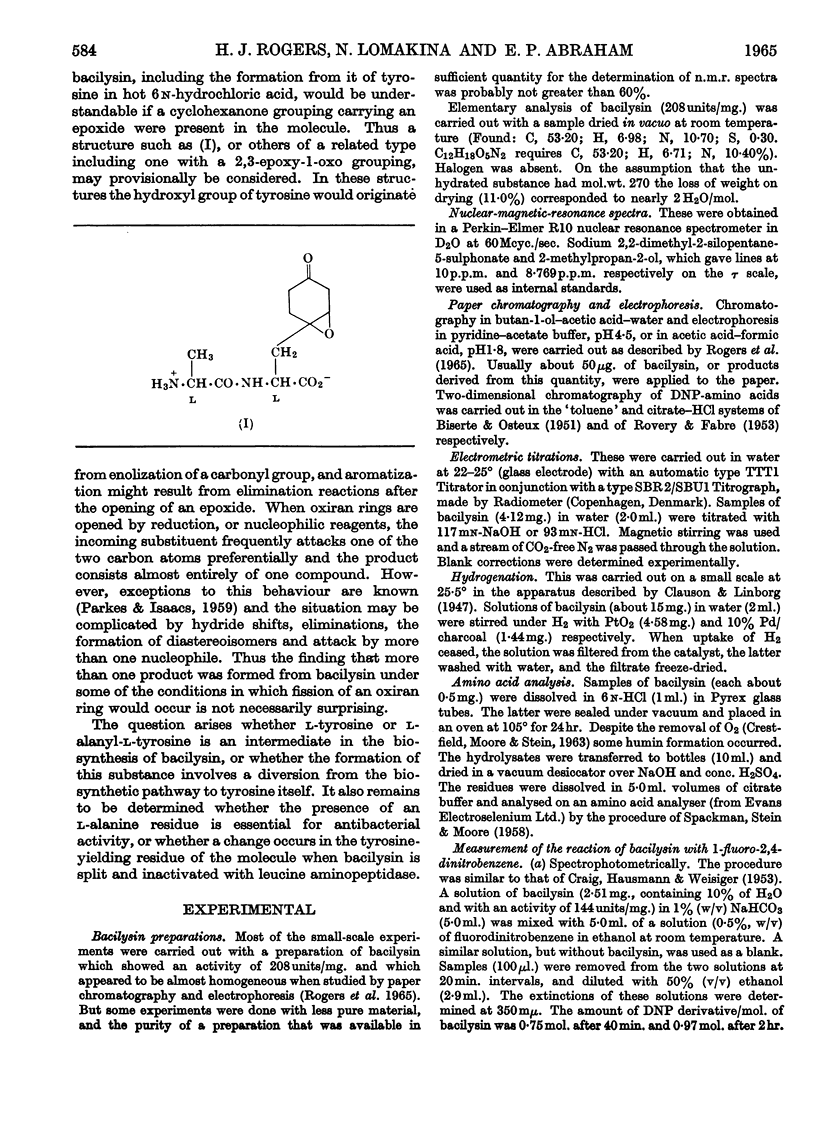
Abstract
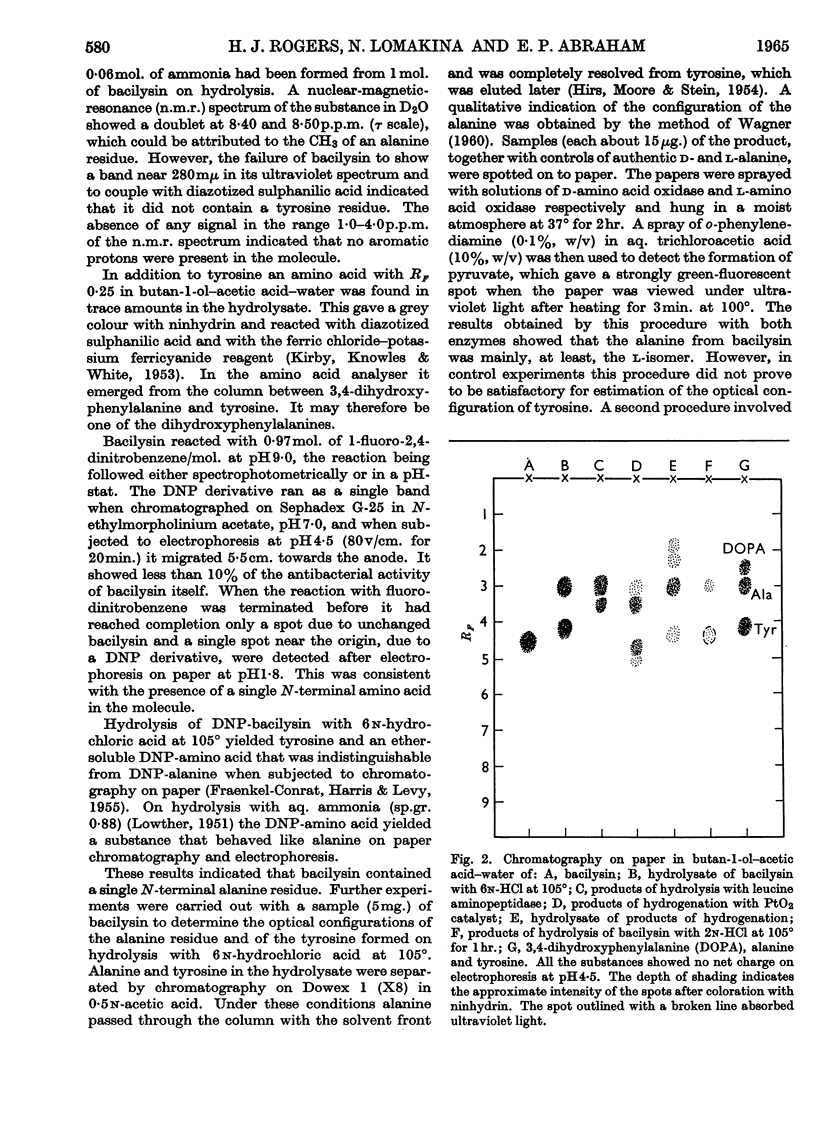
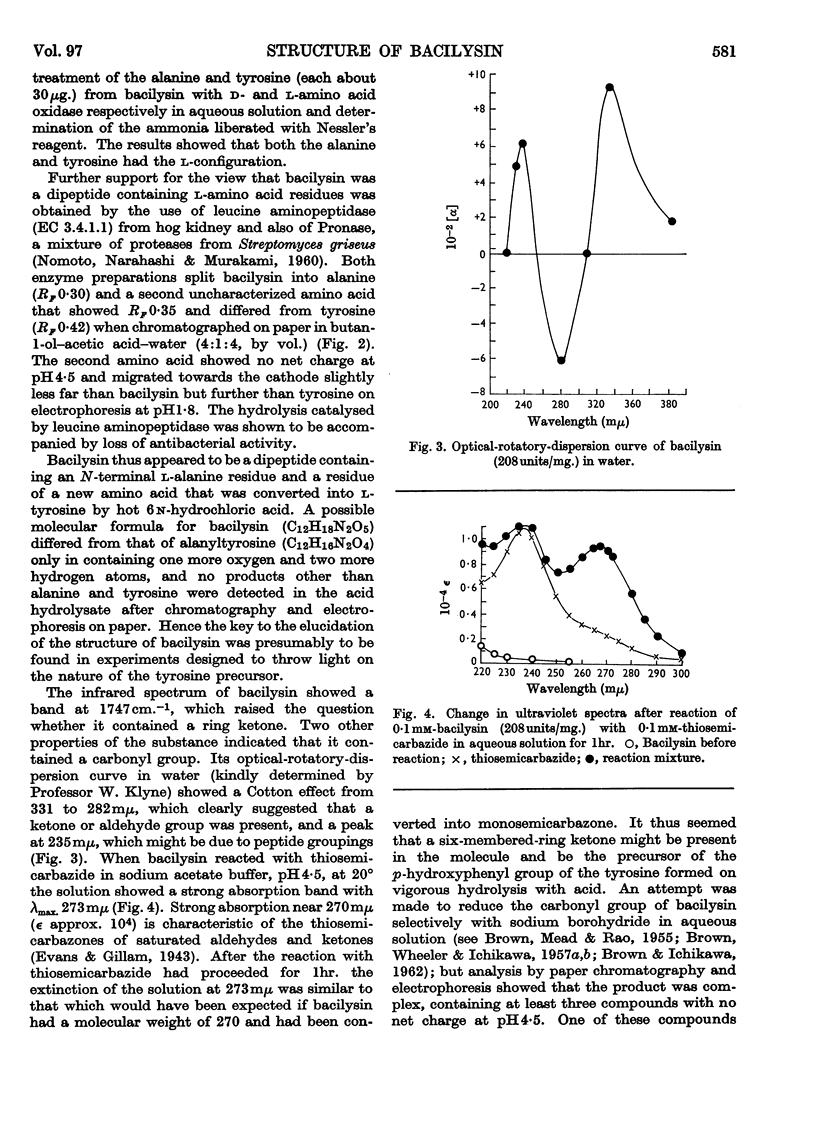
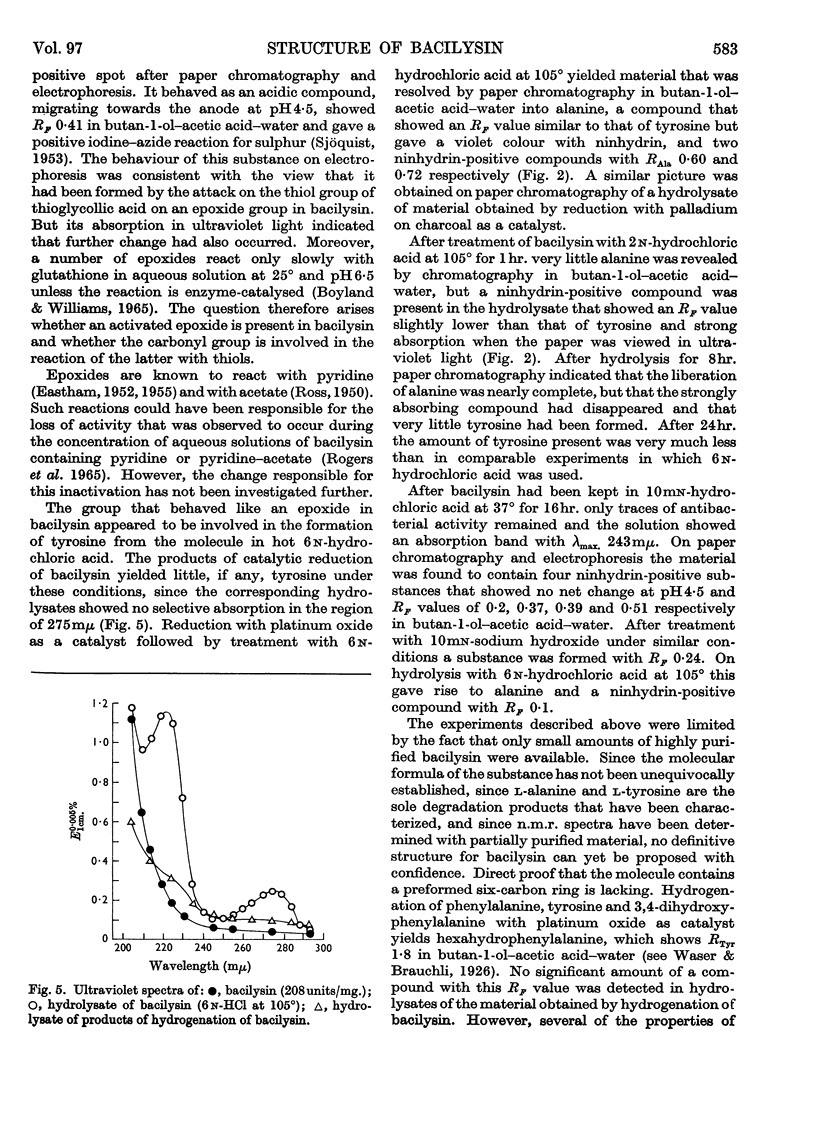
1. Elementary analysis and other properties of a highly purified preparation of bacilysin indicated that a possible molecular formula for the substance is C12H18N2O5. The results of electrometric titration were consistent with the hypothesis that the substance was a peptide containing one free α-amino group and one free carboxyl group. 2. Hydrolysis of bacilysin with 6n-hydrochloric acid at 105° yielded l-alanine and l-tyrosine, but the ultraviolet spectrum of the substance showed that no tyrosine residue was present in the molecule and a nuclear-magnetic-resonance spectrum indicated that olefinic and aromatic protons were absent. The dinitrophenyl (DNP) derivative of bacilysin yielded DNP-alanine on acid hydrolysis. 3. Bacilysin was hydrolysed by leucine aminopeptidase (EC 3.4.1.1) and by Pronase to give alanine and an uncharacterized amino acid. Its infrared spectrum was consistent with the presence of a peptide grouping in the molecule. 4. The optical rotatory dispersion of bacilysin and its reaction with thiosemicarbazide indicated that the substance contained an aldehyde or ketone group. Its behaviour on catalytic reduction and its reaction with sodium thiosulphate and with certain thiols suggested that an epoxide group was present. 5. A possible type of structure for bacilysin is considered in the light of its known properties.
Full text
PDF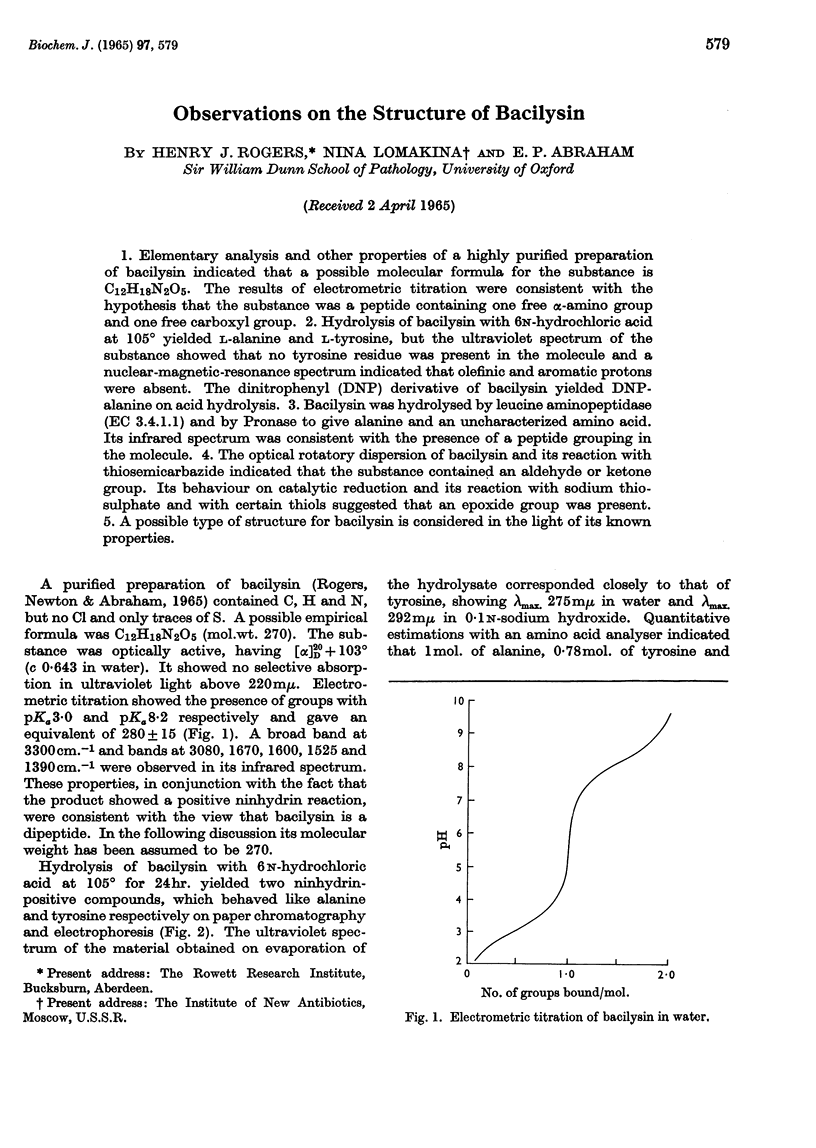



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., WILLIAMS K. AN ENZYME CATALYSING THE CONJUGATION OF EPOXIDES WITH GLUTATHIONE. Biochem J. 1965 Jan;94:190–197. doi: 10.1042/bj0940190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG L. C., HAUSMANN W., WEISIGER J. R. The molecular weight of bacitracin A. J Biol Chem. 1953 Feb;200(2):765–773. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- LOWTHER A. G. Identification of N-2: 4-dinitrophenylamino-acids. Nature. 1951 May 12;167(4254):767–768. doi: 10.1038/167767b0. [DOI] [PubMed] [Google Scholar]
- ROVERY M., FABRE C. Chromatographie sur papier en phase aqueuse pour l'étude des dinitrophenyl (DNP) aminoacides et peptides. Bull Soc Chim Biol (Paris) 1953;35(5-6):541–546. [PubMed] [Google Scholar]
- Rogers H. J., Newton G. G., Abraham E. P. Production and purification of bacilysin. Biochem J. 1965 Nov;97(2):573–578. doi: 10.1042/bj0970573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE F. H., Jr Regeneration of enzymatic activity by airoxidation of reduced ribonuclease with observations on thiolation during reduction with thioglycolate. J Biol Chem. 1960 Feb;235:383–389. [PubMed] [Google Scholar]


