Abstract
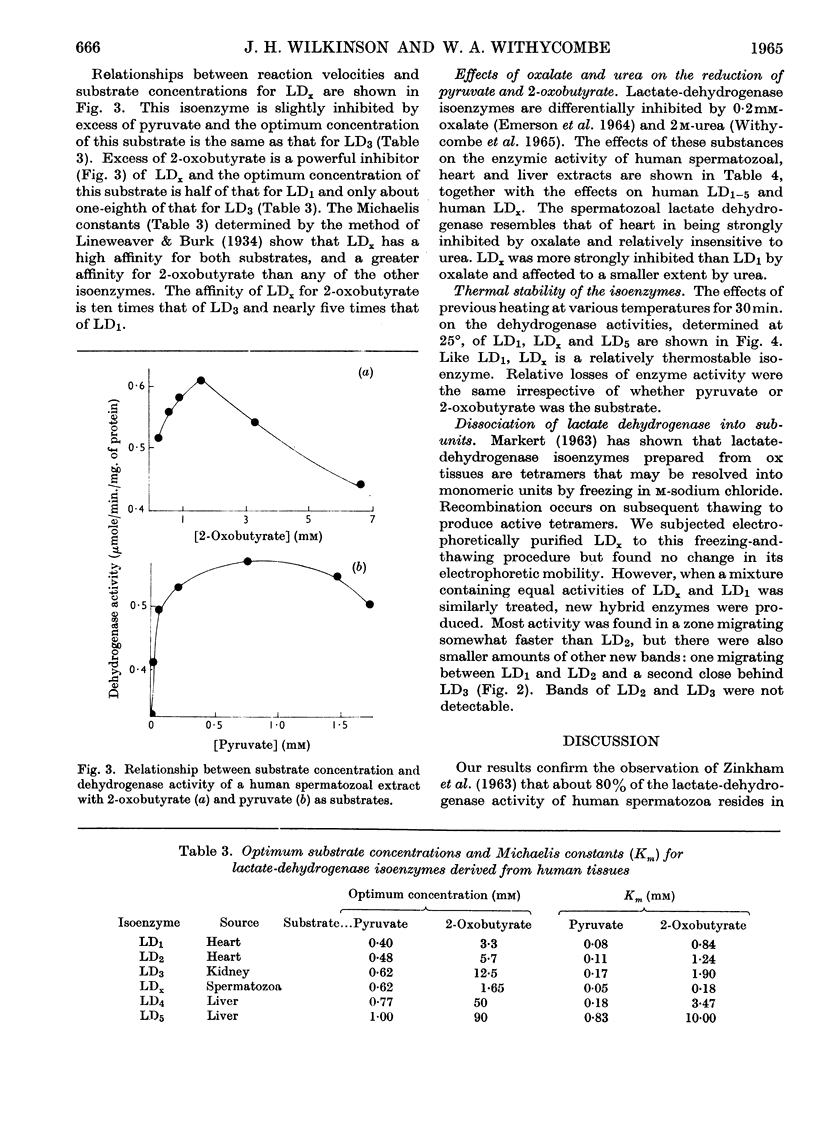
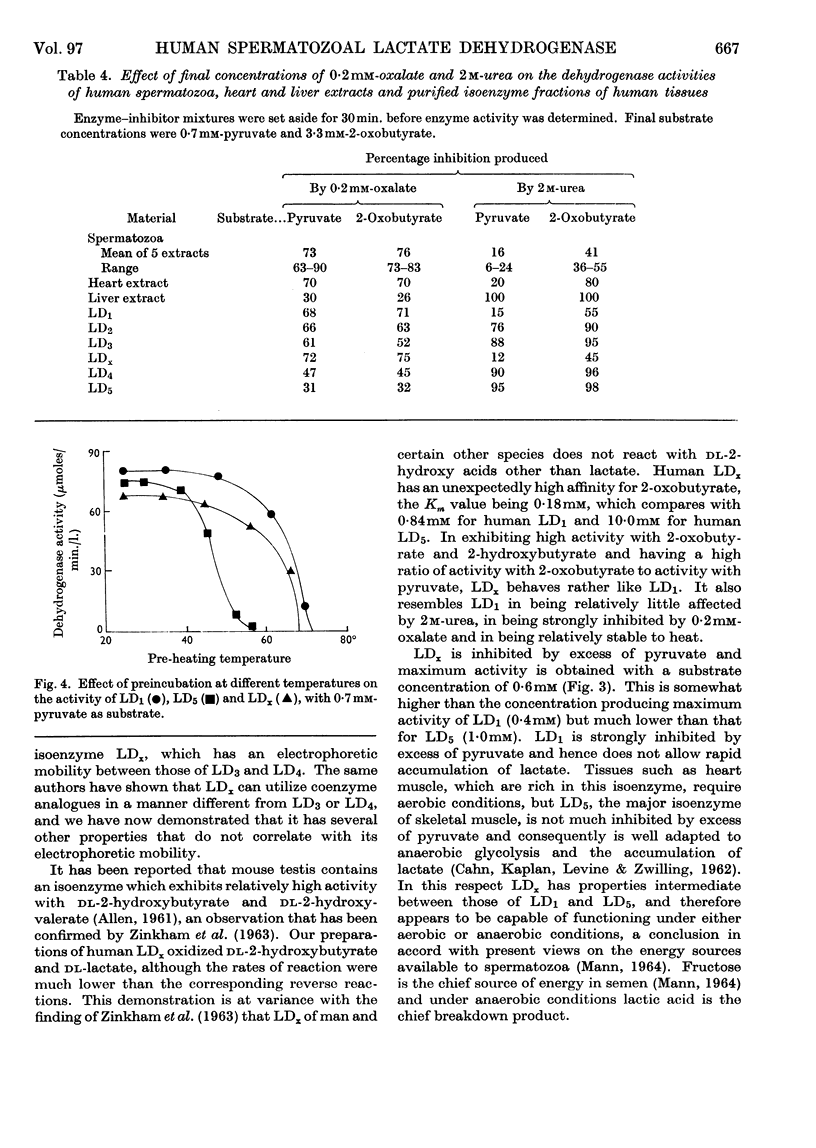
1. The presence of a characteristic lactate-dehydrogenase isoenzyme (LDx) in human, mouse and dog testis and in human spermatozoa has been confirmed by electrophoresis on cellulose acetate and on polyacrylamide gel. 2. The human spermatozoal isoenzyme exhibits a much higher affinity for 2-oxobutyrate than any of the five isoenzymes found in other tissues. Km values of 0·05mm for pyruvate and 0·18mm for 2-oxobutyrate were obtained. 3. LDx differs from other lactate-dehydrogenase isoenzymes in that its properties cannot be correlated with its electrophoretic mobility. It resembles LD1 in being strongly inhibited by 0·2mm-oxalate and relatively resistant to 2m-urea, and in being relatively stable to heat. 4. The surprisingly high activity of LDx with 2-oxobutyrate suggests that this substance or 2-hydroxybutyrate may play a part in spermatozoal metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN J. M. Multiple forms of lactic dehydrogenase in tissues of the mouse: their specificity, cellular localization, and response to altered physiological conditions. Ann N Y Acad Sci. 1961 Nov 2;94:937–951. doi: 10.1111/j.1749-6632.1961.tb35586.x. [DOI] [PubMed] [Google Scholar]
- APPELLA E., MARKERT C. L. Dissociation of lactate dehydrogenase into subunits with guanidine hydrochloride. Biochem Biophys Res Commun. 1961 Nov 20;6:171–176. doi: 10.1016/0006-291x(61)90123-1. [DOI] [PubMed] [Google Scholar]
- Blanco A., Zinkham W. H. Lactate Dehydrogenases in Human Testes. Science. 1963 Feb 15;139(3555):601–602. doi: 10.1126/science.139.3555.601. [DOI] [PubMed] [Google Scholar]
- Cahn R. D., Zwilling E., Kaplan N. O., Levine L. Nature and Development of Lactic Dehydrogenases: The two major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962 Jun 15;136(3520):962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- EMERSON P. M., WILKINSON J. H., WITHYCOMBE W. A. EFFECT OF OXALATE ON THE ACTIVITY OF LACTATE DEHYDROGENASE ISOENZYMES. Nature. 1964 Jun 27;202:1337–1338. doi: 10.1038/2021337a0. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Lactic and Malic Dehydrogenases in Human Spermatozoa. Science. 1963 Feb 15;139(3555):602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Markert C. L. Lactate Dehydrogenase Isozymes: Dissociation and Recombination of Subunits. Science. 1963 Jun 21;140(3573):1329–1330. doi: 10.1126/science.140.3573.1329. [DOI] [PubMed] [Google Scholar]
- Menini E., Norymberski J. K. Determination of oxo steroids as coloured salts of their Girard derivatives. Biochem J. 1964 Oct;93(1):11–14. doi: 10.1042/bj0930011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAGEMANN P. G., GREGORY K. F., WROBLEWSKI F. The electrophoretically distinct forms of mammalian lactic dehydrogenase. 1. Distribution of lactic dehydrogenase. 1. Distribution of lactic dehydrogenases in rabbit and human tissue. J Biol Chem. 1960 Aug;235:2282–2287. [PubMed] [Google Scholar]
- PLUMMER D. T., ELLIOTT B. A., COOKE K. B., WILKINSON J. H. Organ specificity and lactate-dehydrogenase activity. 1. The relative activities with pyruvate and 2-oxobutyrate of electrophoretically separated fractions. Biochem J. 1963 May;87:416–422. doi: 10.1042/bj0870416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER D. T., WILKINSON J. H. Organ specificity and lactatedehydrogenase activity. 2. Some properties of human-heart and liver preparations. Biochem J. 1963 May;87:423–429. doi: 10.1042/bj0870423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND S. A convenient apparatus for vertical gel electrophoresis. Clin Chem. 1962 Sep-Oct;8:455–470. [PubMed] [Google Scholar]
- RAYMOND S., WEINTRAUB L. Acrylamide gel as a supporting medium for zone electrophoresis. Science. 1959 Sep 18;130(3377):711–711. doi: 10.1126/science.130.3377.711. [DOI] [PubMed] [Google Scholar]
- ROSALKI S. B., WILKINSON J. H. Reduction of alpha-ketobutyrate by human serum. Nature. 1960 Dec 24;188:1110–1111. doi: 10.1038/1881110a0. [DOI] [PubMed] [Google Scholar]
- SAYRE F. W., GREENBERG D. M. Purification and properties of serine and threonine dehydrases. J Biol Chem. 1956 Jun;220(2):787–799. [PubMed] [Google Scholar]
- STADTMAN E. R. Symptosium on multiple forms of enzymes and control mechanisms. II. Enzyme multiplicity and function in the regulation of divergent metabolic pathways. Bacteriol Rev. 1963 Jun;27:170–181. doi: 10.1128/br.27.2.170-181.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACKER W. E., DORFMAN L. E. Urinary lactic dehydrogenase activity. I. Screening method for detection of cancer of kidneys and bladder. JAMA. 1962 Sep 15;181:972–978. [PubMed] [Google Scholar]
- WITHYCOMBE W. A., PLUMMER D. T., WILKINSON J. H. ORGAN SPECIFICITY AND LACTATE-DEHYDROGENASE ACTIVITY. DIFFERENTIAL INHIBITION BY UREA AND RELATED COMPOUNDS. Biochem J. 1965 Feb;94:384–389. doi: 10.1042/bj0940384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C. D-Serine dehydrase of Neurospora. J Biol Chem. 1952 Sep;198(1):343–352. [PubMed] [Google Scholar]
- YANOFSKY C., REISSIG J. L. L-Serine dehydrase of Neurospora. J Biol Chem. 1953 Jun;202(2):567–577. [PubMed] [Google Scholar]
- ZINKHAM W. H., BLANCO A., KUPCHYK L. LACTATE DEHYDROGENASE IN TESTIS: DISSOCIATION AND RECOMBINATION OF SUBUNITS. Science. 1963 Dec 6;142(3597):1303–1304. doi: 10.1126/science.142.3597.1303. [DOI] [PubMed] [Google Scholar]


