Abstract
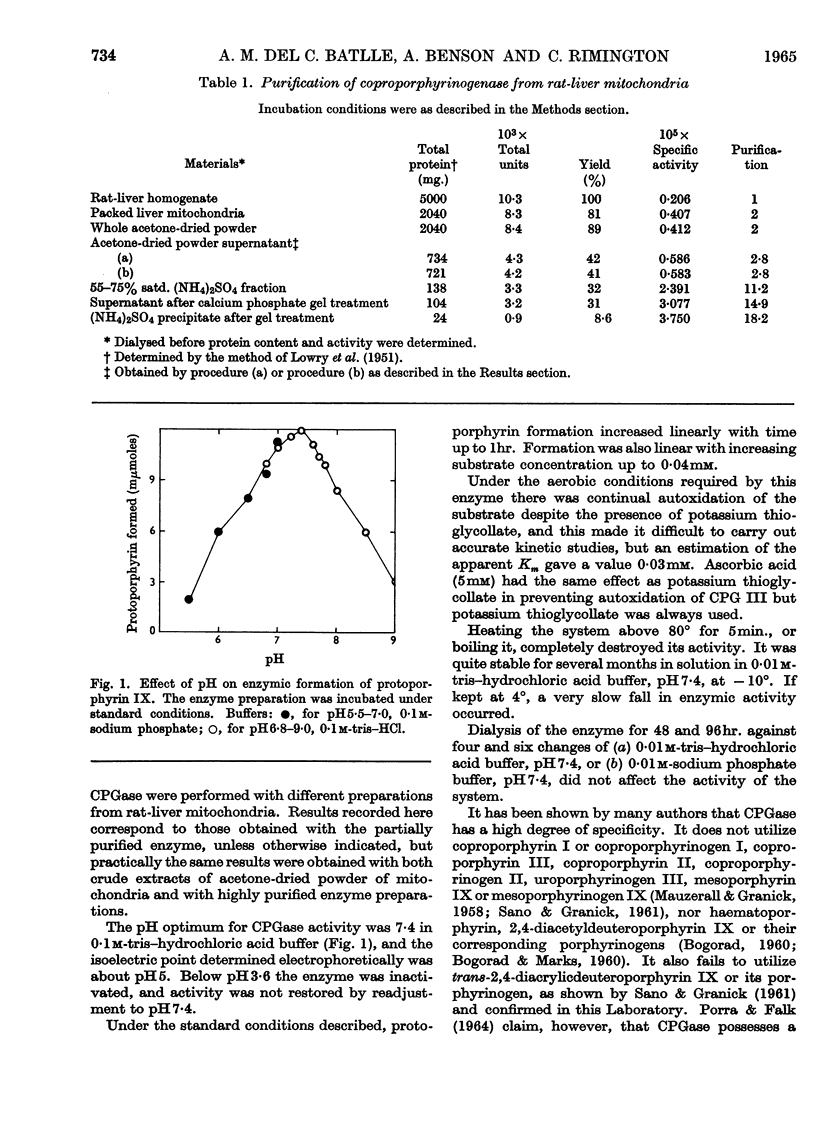
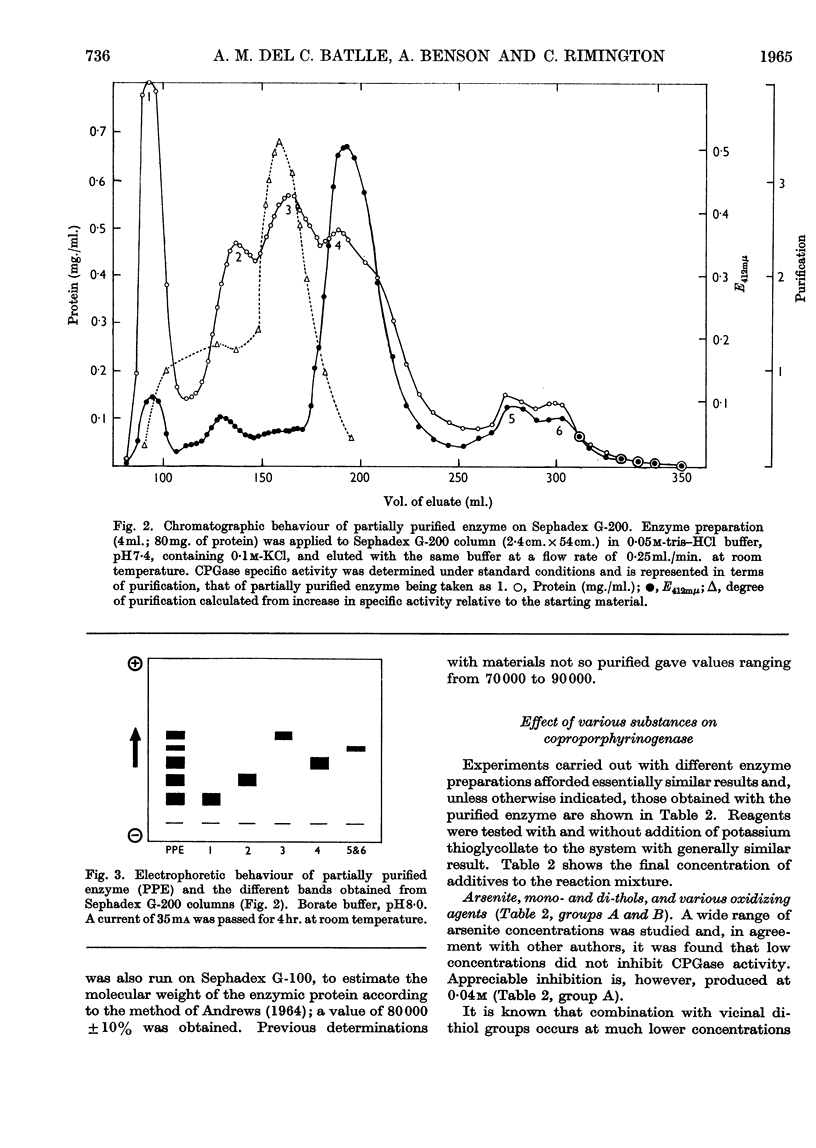
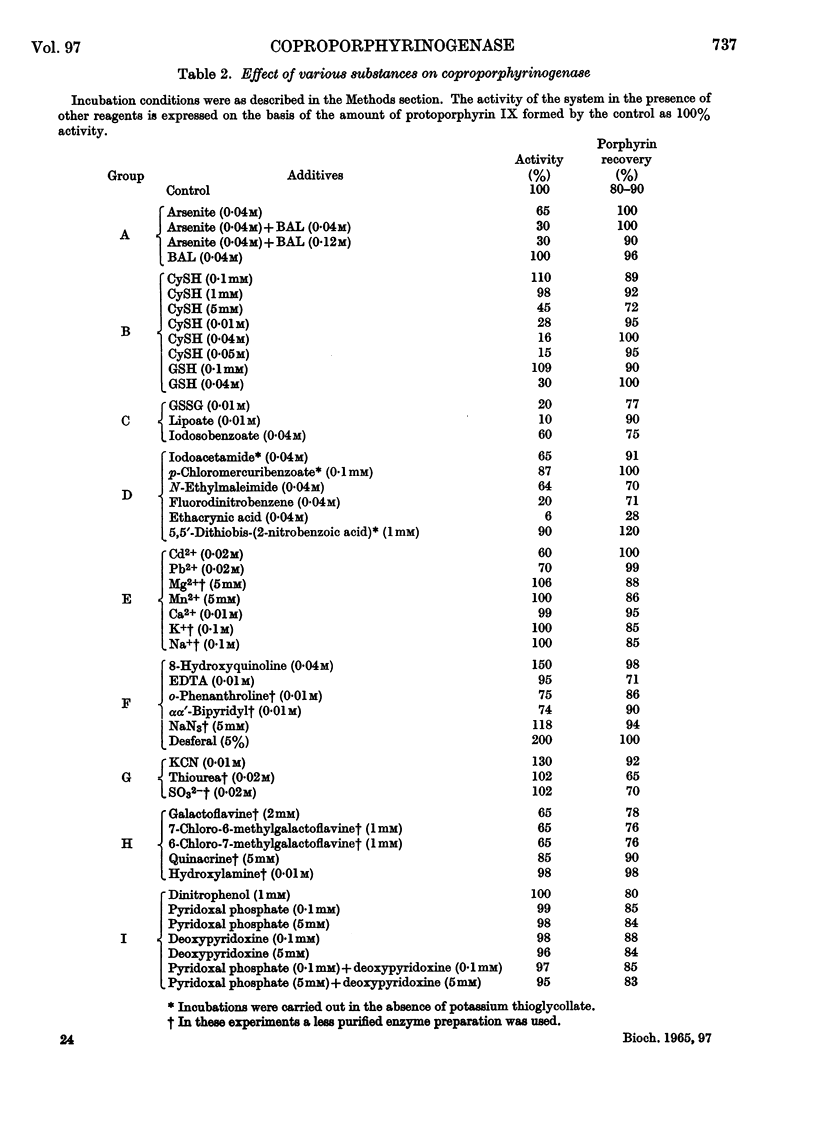
1. Coproporphyrinogenase has been prepared from rat-liver mitochondria and its properties have been studied. The isoelectric point was found to be around pH5·0 and the molecular weight to be 80000±8000. The pH optimum of the enzymic reaction was 7·4 and the apparent Km was of the order 0·03mm. The enzyme was destroyed by boiling and irreversible inactivation occurred below pH3·5. It could be stored at −10° without loss of activity. The enzyme acts specifically on coproporphyrinogen III and does not form protoporphyrinogen from trans-2,4-diacrylicdeuteroporphyrin or its porphyrinogen. It was unaffected by prolonged dialysis and no cofactor requirement could be demonstrated. 2. Column chromatography of a partially purified enzyme preparation on Sephadex G-200 was found to be an improved method of purification, which gave a coproporphyrinogenase 58-fold purified. The purified enzyme was studied electrophoretically but no evidence was obtained to suggest that more than one enzyme was involved in the reaction. 3. The action was studied of various compounds added to the system. The presence of monothiol groups in the enzyme system was indicated, whereas vicinal dithiol groups were not involved in the reaction. Metal-chelating agents did not inhibit the reaction and no requirement for the presence of any essential metal has been found. All attempts to demonstrate the presence of a prosthetic group, in particular flavines, failed. Neither pyridoxal phosphate nor ATP was involved in the reaction, nor was a mitochondrial electron-transport chain required for the activity of the enzyme. Some circumstantial evidence was obtained to suggest that cis-2,4-diacrylicdeuteroporphyrin is an intermediate in the reaction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGORAD L., MARKS G. S. The enzymatic synthesis of uroporphyrins from porphobilinogen. IV. Investigations on the participation of formaldehyde. J Biol Chem. 1960 Jul;235:2127–2129. [PubMed] [Google Scholar]
- Cornford P. Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64–73. doi: 10.1042/bj0910064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. A nomogram for ammonium sulphate solutions. Biochem J. 1953 Jun;54(3):457–458. doi: 10.1042/bj0540457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESEL E. I., FALK J. E. Studies on the biosynthesis of blood pigments. I. Haem synthesis in haemolysed erythrocytes of chicken blood. Biochem J. 1954 Jan;56(1):156–163. doi: 10.1042/bj0560156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSEN L. Paper chromatographic separation of the coproporphyrin isomers I and III. Scand J Clin Lab Invest. 1958;10(3):319–322. [PubMed] [Google Scholar]
- FALK J. E., BENSON A. Separation of uroporphyrin esters I and III by paper chromatography. Biochem J. 1953 Aug;55(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- FALK J. E., DRESEL E. I., RIMINGTON C. Porphobilinogen as a porphyrin precursor, and interconversion of porphyrins, in a tissue system. Nature. 1953 Aug 15;172(4372):292–294. doi: 10.1038/172292a0. [DOI] [PubMed] [Google Scholar]
- GRANICK S., LEVERE R. D. HEME SYNTHESIS IN ERYTHROID CELLS. Prog Hematol. 1964;4:1–47. [PubMed] [Google Scholar]
- Gray C. H. The isolation of coproporphyrin III from Corynebacterium diphtheriae culture filtrates. Biochem J. 1948;43(2):191–193. [PMC free article] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Purification of horse-radish peroxidase and comparison of its properties with those of catalase and methaemoglobin. Biochem J. 1951 Jun;49(1):88–104. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORN R. M., CAFRUNY E. J. ETHACRYNIC ACID: DIURETIC PROPERTY COUPLED TO REACTION WITH SULFHYDRYL GROUPS OF RENAL CELLS. Science. 1964 Jan 10;143(3602):133–134. doi: 10.1126/science.143.3602.133. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. Porphyrin biosynthesis in erythrocytes. III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958 Jun;232(2):1141–1162. [PubMed] [Google Scholar]
- MORTON R. K. Structure and properties of the flavohaemoprotein, cytochrome b2 (L-lactate dehydrogenase of baker's yeast) and of haemoprotein, flavoprotein and apoprotein derivatives. Nature. 1961 Nov 25;192:727–731. doi: 10.1038/192727a0. [DOI] [PubMed] [Google Scholar]
- Morgan E. J., Friedmann E. Maleic acid as inhibitor of enzyme reactions induced by SH-compounds. Biochem J. 1938 May;32(5):862–870. doi: 10.1042/bj0320862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., FALK J. E. Protein-bound porphyrins associated with protoporphyrin biosynthesis. Biochem Biophys Res Commun. 1961 Jun 28;5:179–184. doi: 10.1016/0006-291x(61)90106-1. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIMINGTON C., SVEINSSON S. L. The spectrophotometric determination of uroporphyrin. Scand J Clin Lab Invest. 1950;2(3):209–216. doi: 10.3109/00365515009049872. [DOI] [PubMed] [Google Scholar]
- RIMINGTON C., TOOTH B. E. Role of mitochondria in the in vitro formation of protoporphyrin and haem. J Biochem. 1961 Jun;49:456–467. doi: 10.1093/oxfordjournals.jbchem.a127328. [DOI] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- SEAMAN G. R., NASCHKE M. D. Removal of thioctic acid from enzymes. J Biol Chem. 1955 Apr;213(2):705–711. [PubMed] [Google Scholar]
- WILDY J., NIZET A., BENSON A. Identification of a plasma material stimulating haemoglobin synthesis in vitro. Biochim Biophys Acta. 1961 Dec 23;54:414–423. doi: 10.1016/0006-3002(61)90080-4. [DOI] [PubMed] [Google Scholar]


