Abstract
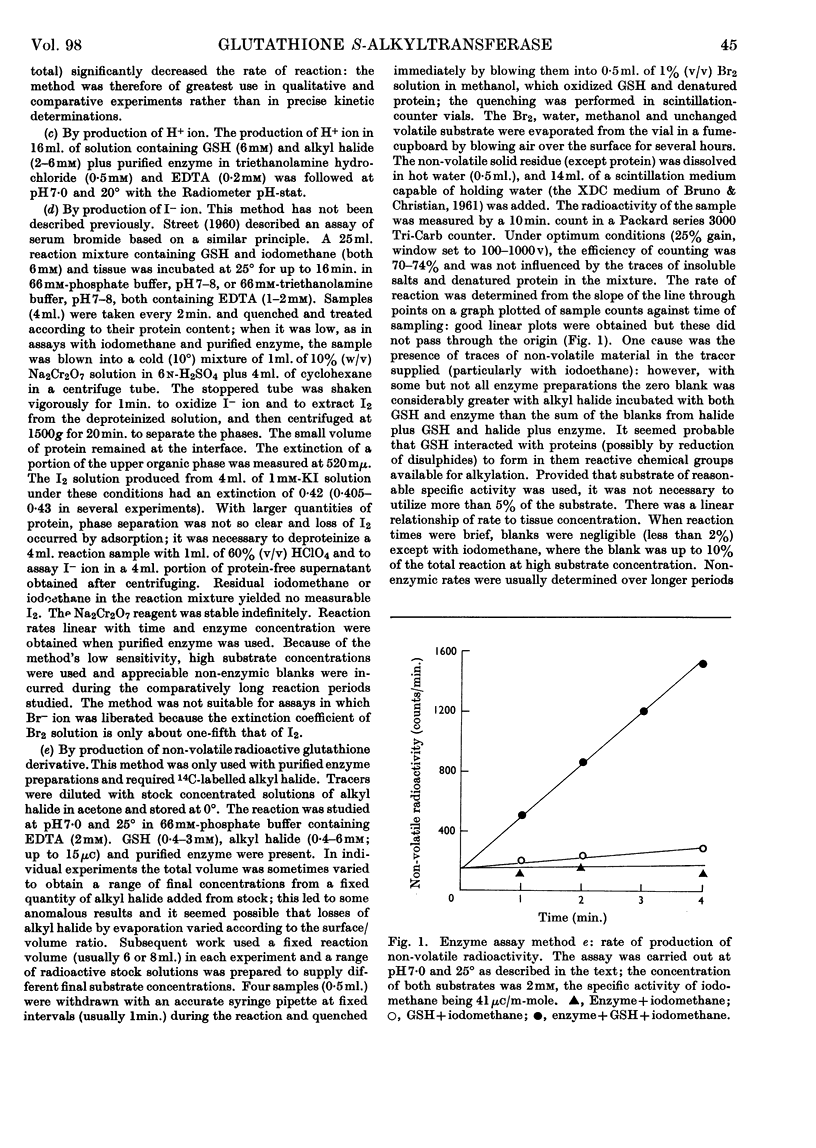
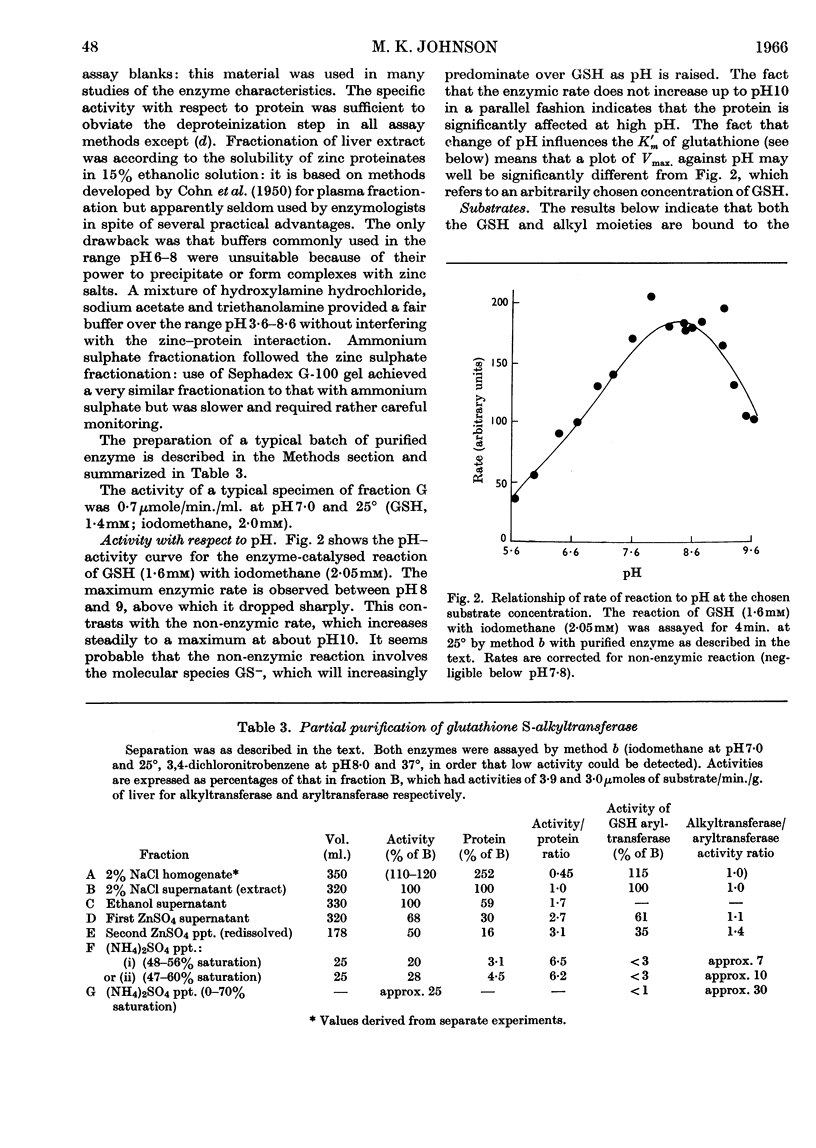
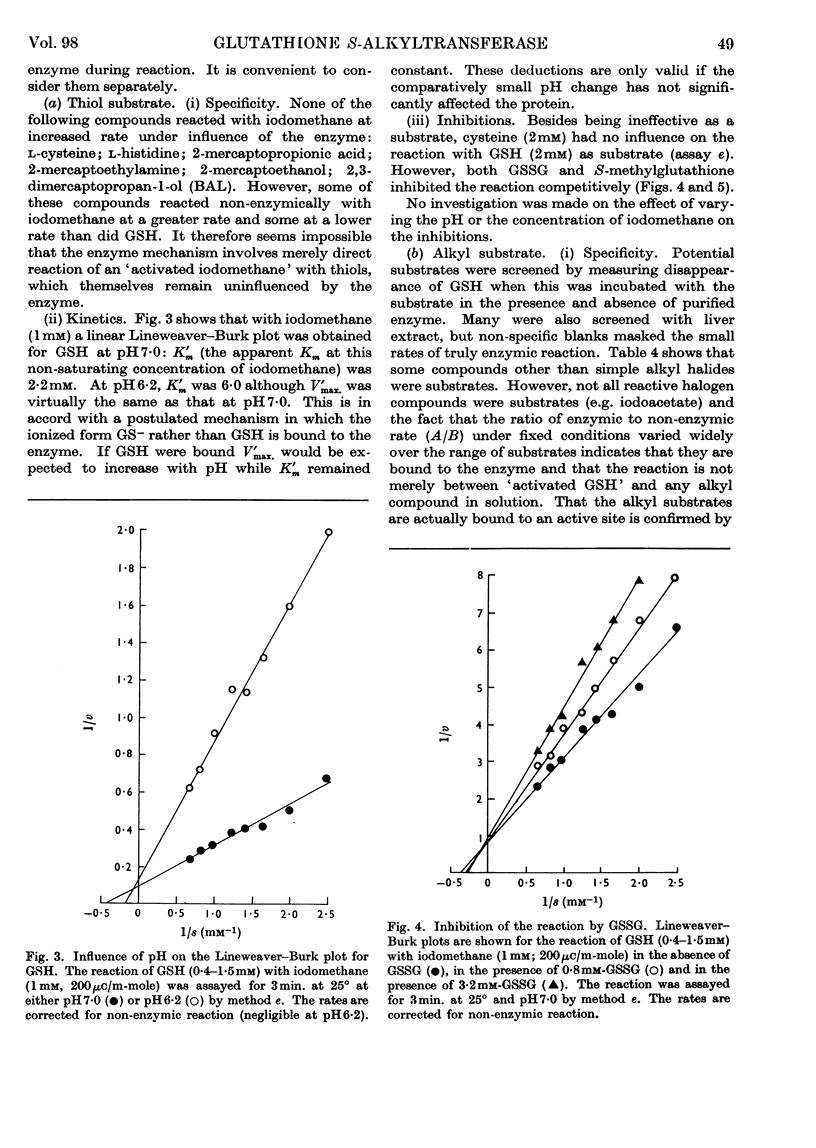
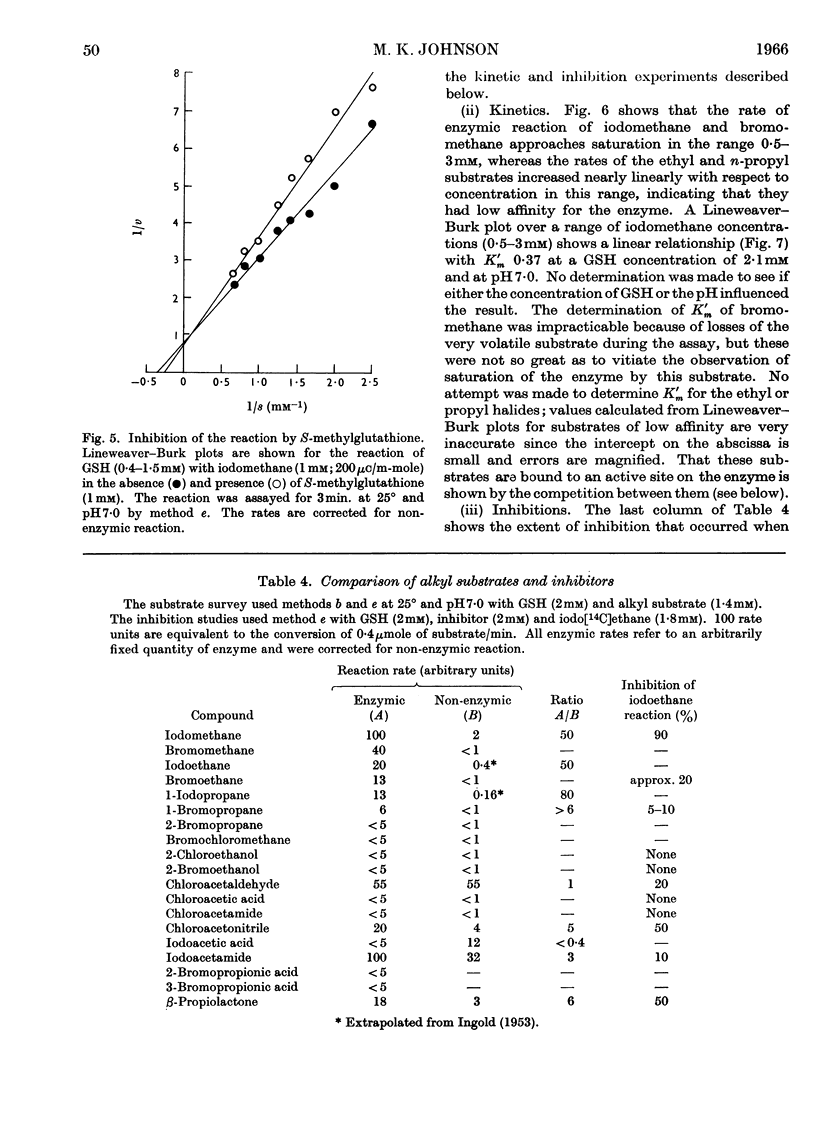
1. A rat-liver enzyme catalysing the S-alkylation of glutathione by iodomethane and various other alkyl compounds has been identified and partially purified; its stability, specificity and response to inhibitors and activators and to changes in reaction pH have been studied. 2. The enzyme is distinct from glutathione S-aryltransferase, but both enzymes respond similarly to various inhibitors. 3. A similar enzyme has been found in the kidney and adrenal of rat and in the liver and kidney of numerous species. 4. The identity and the physiological role of the enzyme are discussed.
Full text
PDF


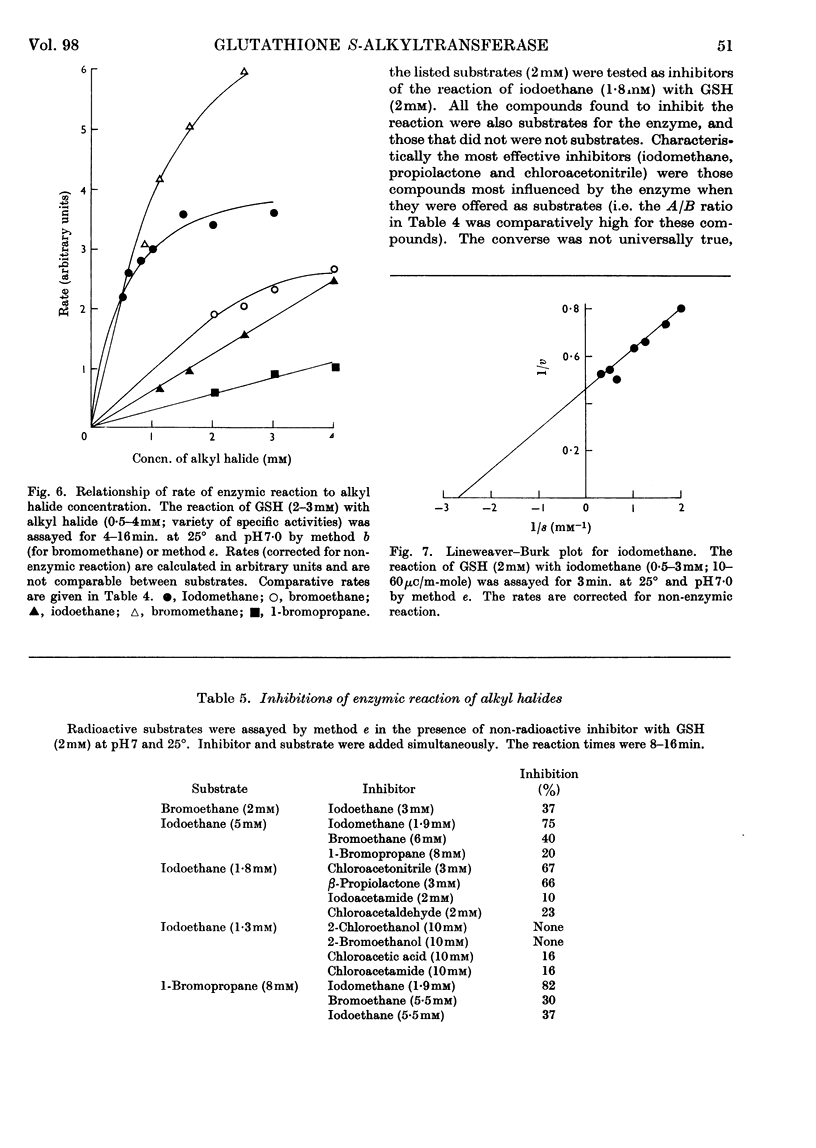
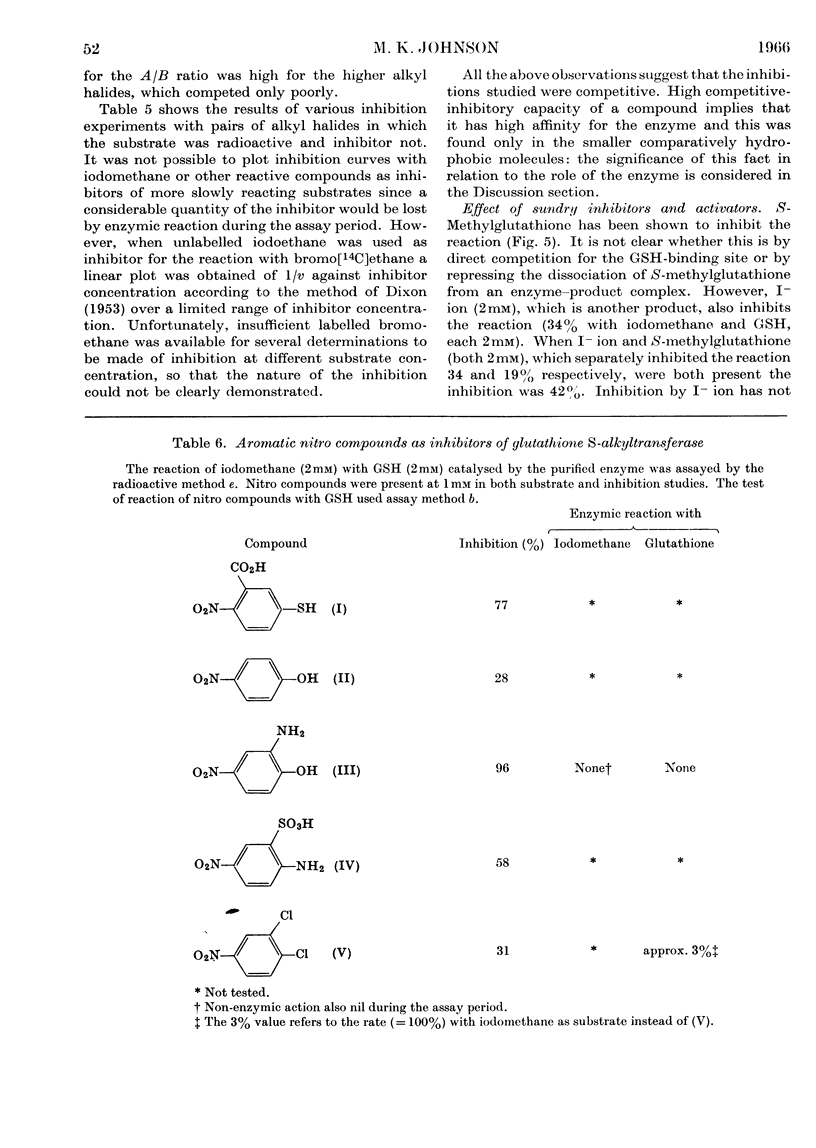




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AL-KASSAB S., BOYLAND E., WILLIAMS K. An enzyme from rat liver catalysing conjugations with glutathione. 2. Replacement of nitro groups. Biochem J. 1963 Apr;87:4–9. doi: 10.1042/bj0870004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N., EMERY R. C., STREET B. W. A tissue homogenizer. Biochem J. 1960 Nov;77:326–327. doi: 10.1042/bj0770326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYLAND E., WILLIAMS K. AN ENZYME CATALYSING THE CONJUGATION OF EPOXIDES WITH GLUTATHIONE. Biochem J. 1965 Jan;94:190–197. doi: 10.1042/bj0940190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMBES B., STAKELUM G. S. A liver enzyme that conjugates sulfobromophthalein sodium with glutathione. J Clin Invest. 1961 Jun;40:981–988. doi: 10.1172/JCI104337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FODOR P. J., MILLER A., WAELSCH H. Quantitative aspects of enzymatic cleavage of glutathione. J Biol Chem. 1953 Jun;202(2):551–565. [PubMed] [Google Scholar]
- FROMAGEOT P., SENTENAC A. BETA SUBSTITUTION ENZYMATIQUE DE LA PHOSPHOS'ERINE ET DE LA CYST'EINE PAR LE SULFITE. QUELQUES REMARQUES SUR LE M'ECANISME DE LA BETA SUBSTITUTION EN PR'ESENCE D'ENZYMES 'A PHOSPHATE DE PYRIDOXAL. J Biochem. 1964 Jun;55:659–668. [PubMed] [Google Scholar]
- GOLDBARG J. A., FRIEDMAN O. M., PINEDA E. P., SMITH E. E., CHATTERJI R., STEIN E. H., RUTENBURG A. M. The colorimetric determination of gamma-glutamyl transpeptidase with a synthetic substrate. Arch Biochem Biophys. 1960 Nov;91:61–70. doi: 10.1016/0003-9861(60)90455-0. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Conjugations with glutathione. Distribution of glutathione S-aryltransferase in vertebrate species. Biochem J. 1964 Mar;90(3):603–606. doi: 10.1042/bj0900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Metabolism of iodomethane in the rat. Biochem J. 1966 Jan;98(1):38–43. doi: 10.1042/bj0980038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZEL P., HENDERSON J. F. ON THE RELATIONSHIP BETWEEN LIPID SOLUBILITY AND MICROSOMAL METABOLISM OF DRUGS. Biochem Pharmacol. 1965 Jan 1;14:92–94. doi: 10.1016/0006-2952(65)90063-8. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Glutathione peroxidase and the destruction of hydrogen peroxide in animal tissues. Arch Biochem Biophys. 1960 Jan;86:1–5. doi: 10.1016/0003-9861(60)90357-x. [DOI] [PubMed] [Google Scholar]
- MILLS G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957 Nov;229(1):189–197. [PubMed] [Google Scholar]
- NARAHARA H. T., WILLIAMS R. H. Reduction of insulin by extracts of rat liver. J Biol Chem. 1959 Jan;234(1):71–77. [PubMed] [Google Scholar]
- NEUBERT D., ROSE T. H., LEHNINGER A. L. Assay and cellular distribution of mitochondrial "contraction factor". J Biol Chem. 1962 Jun;237:2025–2031. [PubMed] [Google Scholar]
- NEUBERT D., WOJTCZAK A. B., LEHNINGER A. L. Purification and enzymatic identity of mitochondrial contraction-factors I and II. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1651–1658. doi: 10.1073/pnas.48.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Glutathione-homocystine transhydrogenase. J Biol Chem. 1955 Dec;217(2):867–874. [PubMed] [Google Scholar]
- RALL T. W., LEHNINGER A. L. Glutathione reductase of animal tissues. J Biol Chem. 1952 Jan;194(1):119–130. [PubMed] [Google Scholar]
- REVEL J. P., BALL E. G. The reaction of glutathione with amino acids and related compounds as catalyzed by gamma-glutamyl transpeptidase. J Biol Chem. 1959 Mar;234(3):577–582. [PubMed] [Google Scholar]
- RODNIGHT R., McILWAIN H. Techniques in tissue metabolism. III. Study of tissue fragments with little or no added aqueous phase, and in oils. Biochem J. 1954 Aug;57(4):649–661. doi: 10.1042/bj0570649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUROG A., BRIGGS F. N., CHAIKOFF I. L. I131-labeled 1-thyroxine. II. Nature of the excretion product in bile. J Biol Chem. 1952 Feb;194(2):655–668. [PubMed] [Google Scholar]
- TOMIZAWA H. H., HALSEY Y. D. Isolation of an insulin-degrading enzyme from beef liver. J Biol Chem. 1959 Feb;234(2):307–310. [PubMed] [Google Scholar]



