Abstract
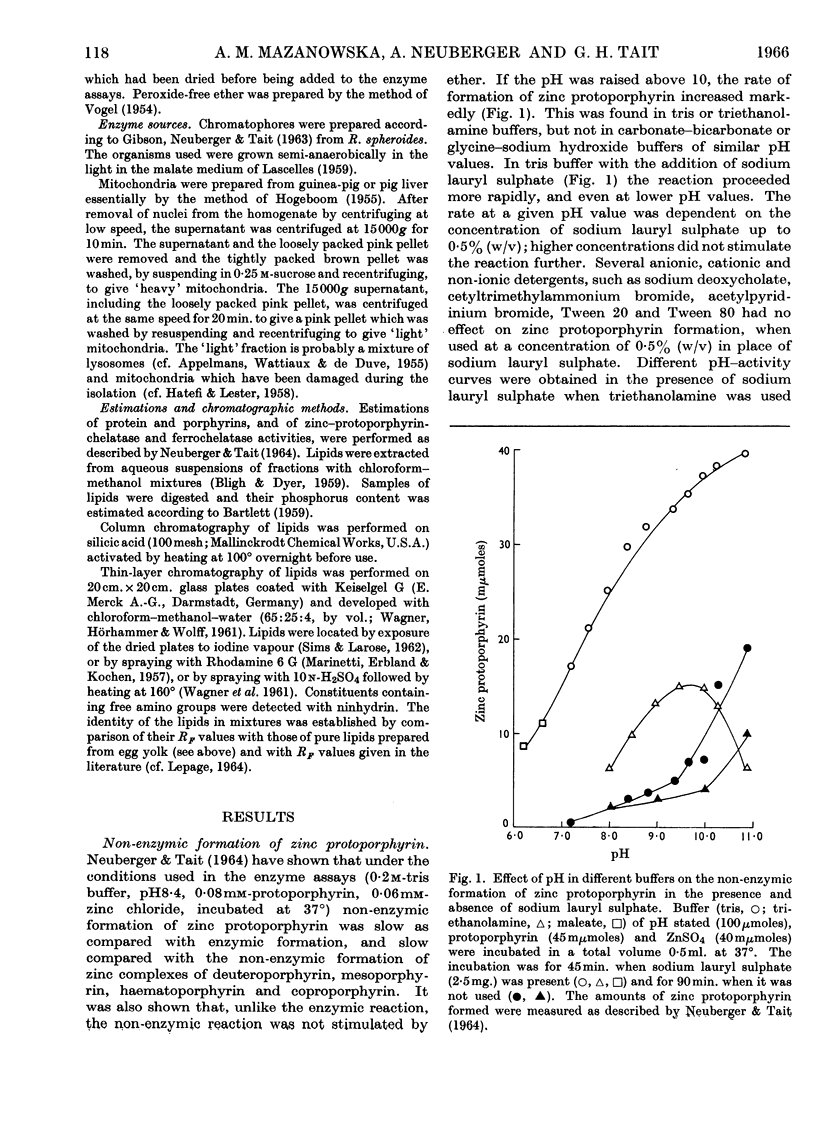
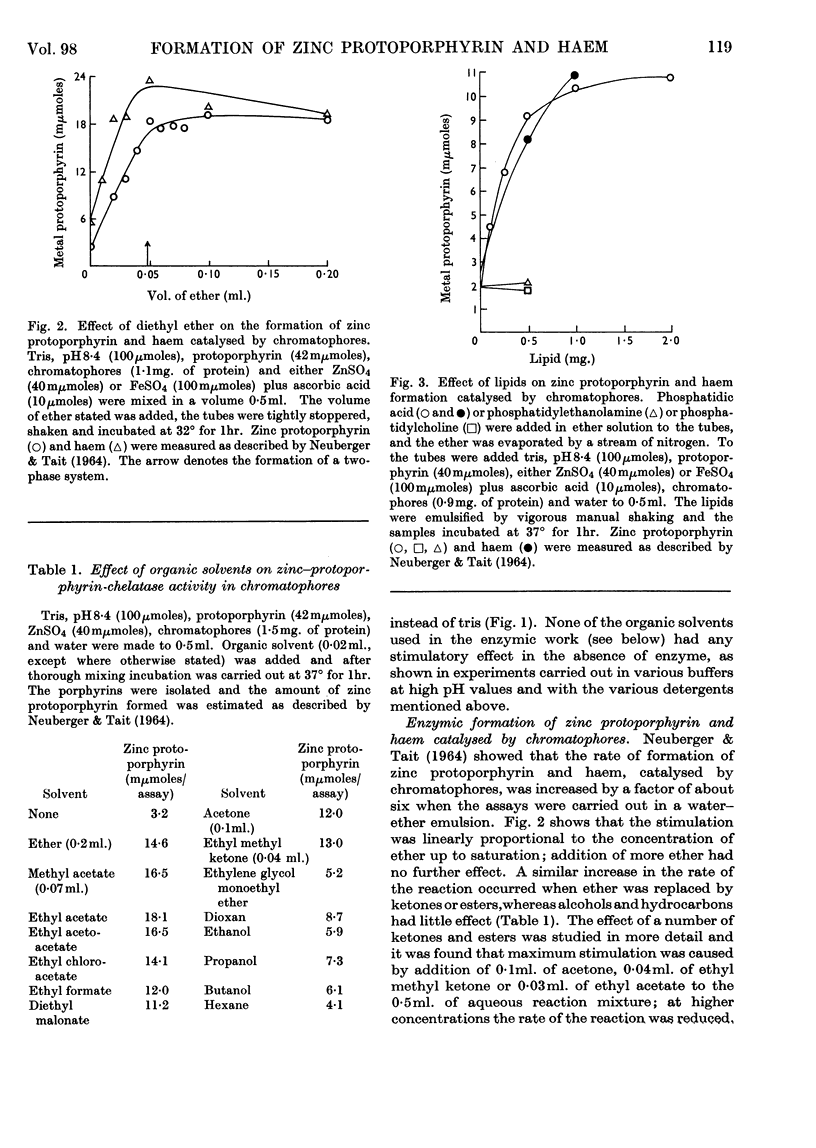
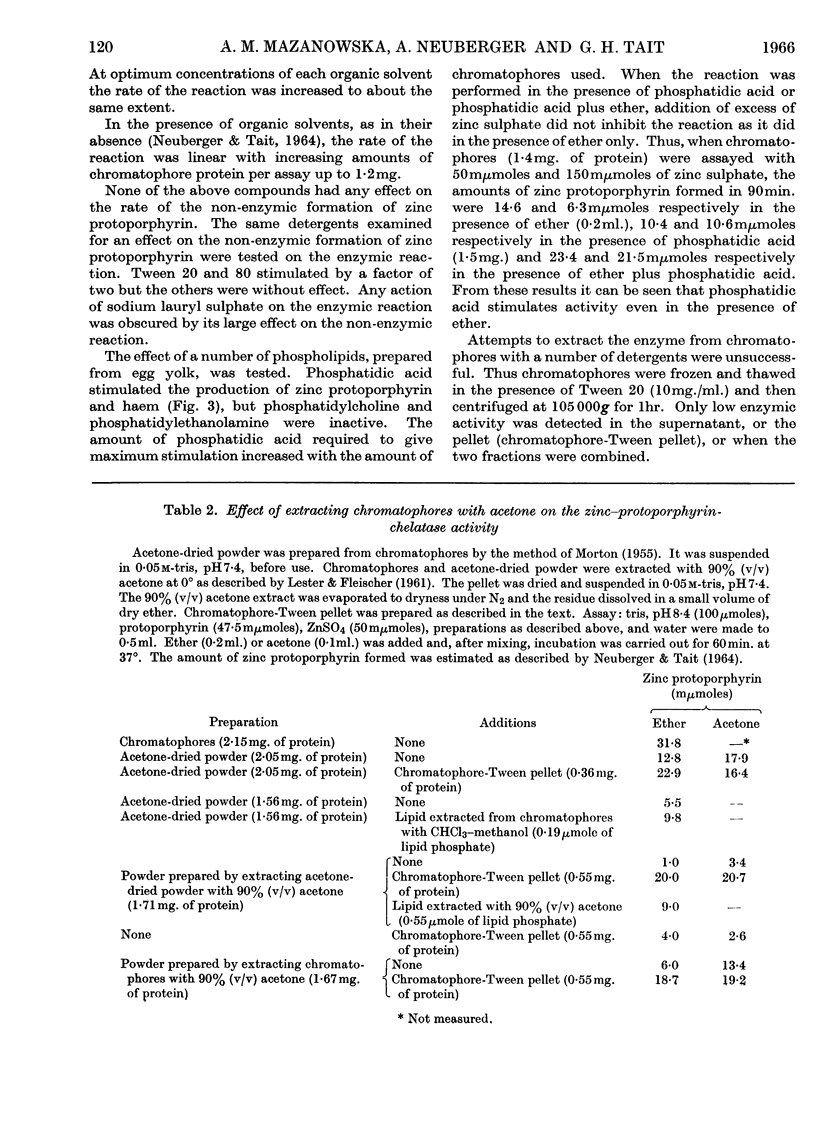
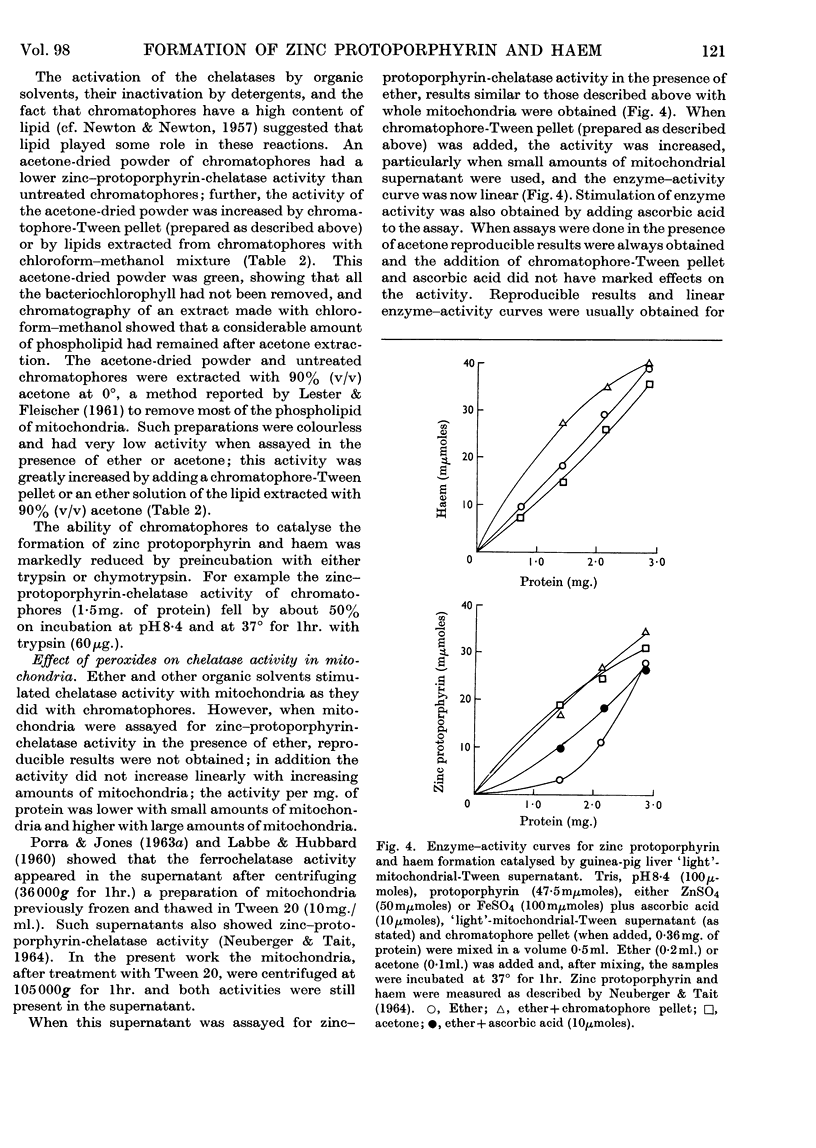
1. Differences observed in earlier work between the enzymic chelation with protoporphyrin of Zn2+ and Fe2+ ions respectively have now been explained as being caused by the presence of peroxides in the ether used in the enzyme assay. The inhibitory effect of peroxides is established by the reducing agent, which is present in the assay for chelation of iron but not in that for zinc. There are now no reasons for the belief that two different enzymes catalyse formation of complexes with zinc and iron respectively. 2. Removal of lipid from both chromatophores and mitochondria markedly reduced chelatase activity. Activity could be partially restored by the addition of lipid fractions. Phosphatidic acid, but not phosphatidylcholine or phosphatidylethanolamine, actively stimulated the formation of zinc protoporphyrin and haem by chromatophores and mitochondrial preparations. 3. Lipid-containing extracts of chromatophores, and fractions thereof obtained by silicic acid chromatography, partially restored chelatase activity of Tween extracts of mitochondria. Thus, although both enzymes are considered to be lipoproteins, the identity of the lipids concerned is still uncertain. 4. A great number of organic solvents such as esters, ethers, ketones and, to a lesser extent, alcohols, stimulate enzymic chelation of both metals with protoporphyrin. A number of explanations for these findings are considered and it is suggested that organic solvents interact in some way with the enzyme lipoprotein, changing either its conformation or allowing closer contact between the enzyme and its substrates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASANO A., KANESHIRO T., BRODIE A. F. MALATE-VITAMIN K REDUCTASE, A PHOSPHOLIPID-REQUIRING ENZYME. J Biol Chem. 1965 Feb;240:895–905. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. ON THE MECHANISM OF ACTION OF PHOSPHOLIPASE A. Biochem J. 1963 Sep;88:414–423. doi: 10.1042/bj0880414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORCHEIN A. ORNITHINE IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1964 Jun 15;84:356–358. doi: 10.1016/0926-6542(64)90064-2. [DOI] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- HANAHAN D. J., RODBELL M., TURNER L. D. Enzymatic formation of monopalmitoleyl- and monopalmitoyllecithin (lysolecithins). J Biol Chem. 1954 Jan;206(1):431–441. [PubMed] [Google Scholar]
- HANAHAN D. J. The enzymatic degradation of phosphatidyl choline in diethyl ether. J Biol Chem. 1952 Mar;195(1):199–206. [PubMed] [Google Scholar]
- HATEFI Y., LESTER R. L. Studies on the mechanism of oxidative phosphorylation. III. Phosphorylating particle types from beef heart. Biochim Biophys Acta. 1958 Jan;27(1):83–88. doi: 10.1016/0006-3002(58)90294-4. [DOI] [PubMed] [Google Scholar]
- JURTSHUK P., Jr, SEKUZU I., GREEN D. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVI. ON THE FORMATION OF AN ACTIVE COMPLEX BETWEEN THE APO-D(--)-BETA-HYDROXYBUTYRIC DEHYDROGENASE AND MICELLAR LECITHIN. J Biol Chem. 1963 Nov;238:3595–3605. [PubMed] [Google Scholar]
- KATES M. Effects of solvents and surface-active agents on plastid phosphatidase C activity. Can J Biochem Physiol. 1957 Feb;35(2):127–142. [PubMed] [Google Scholar]
- KATES M. Hydrolysis of lecithin by plant plastid enzymes. Can J Biochem Physiol. 1955 Jul;33(4):575–589. [PubMed] [Google Scholar]
- KATES M. Lecithinase activity of chloroplasts. Nature. 1953 Oct 31;172(4383):814–815. doi: 10.1038/172814a0. [DOI] [PubMed] [Google Scholar]
- LABBE R. F. An enzyme which catalyzes the insertion of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):589–590. doi: 10.1016/0006-3002(59)90053-8. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Preparation and properties of the iron-protoporphyrin chelating enzyme. Biochim Biophys Acta. 1960 Jul 1;41:185–191. doi: 10.1016/0006-3002(60)90001-9. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., FLEISCHER S. Studies on the electron-transport system. 27. The respiratory activity of acetoneextracted beef-heart mitochondria: role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Feb 18;47:358–377. doi: 10.1016/0006-3002(61)90297-9. [DOI] [PubMed] [Google Scholar]
- LOWE M. B., PHILLIPS J. N. Catalysis of metalloporphyrin formation: a possible enzyme model for haem iron incorporation. Nature. 1961 Apr 15;190:262–263. doi: 10.1038/190262a0. [DOI] [PubMed] [Google Scholar]
- MAGEE W. L., THOMPSON R. H. The estimation of phospholipase A activity in aqueous systems. Biochem J. 1960 Dec;77:526–534. doi: 10.1042/bj0770526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINETTI G. V., ERBLAND J., KOCHEN J. Quantitative chromatography of phosphatides. Fed Proc. 1957 Sep;16(3):837–844. [PubMed] [Google Scholar]
- NEWTON J. W., NEWTON G. A. Composition of the photoactive subcellular particles from Chromatium. Arch Biochem Biophys. 1957 Sep;71(1):250–265. doi: 10.1016/0003-9861(57)90026-7. [DOI] [PubMed] [Google Scholar]
- Neuberger A., Tait G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 5. Zinc-protoporphyrin chelatase. Biochem J. 1964 Mar;90(3):607–616. doi: 10.1042/bj0900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PESCH L. A., PETERSON J. PHOSPHOLIPID--PROTEIN INTERACTION AS A DETERMINANT FOR THE SUBSTRATE SPECIFICITY OF MITOCHONDRIAL NICOTINAMIDE--ADENINE-DINUCLEOTIDE (PHOSPHATE) TRANSHYDROGENASE. Biochim Biophys Acta. 1965 Mar 22;96:390–394. doi: 10.1016/0005-2787(65)90559-9. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobari J. Requirement of flavin adenine dinucleotide and phospholipid for the activity of malate dehydrogenase from Mycobacterium avium. Biochem Biophys Res Commun. 1964 Feb 18;15(1):50–54. doi: 10.1016/0006-291x(64)90101-9. [DOI] [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WARD H. A., FANTL P. Transfer of hydrophilic cations from an aqueous to a lipophilic phase by phosphatidic acids. Arch Biochem Biophys. 1963 Feb;100:338–340. doi: 10.1016/0003-9861(63)90083-3. [DOI] [PubMed] [Google Scholar]
- WOJTCZAK L., WLODAWER P., ZBOROWSKI J. Adenosine triphosphate-indeced contraction of rat-liver mitochondria and synthesis of mitochondrial phospholipids. Biochim Biophys Acta. 1963 Jun 18;70:290–305. doi: 10.1016/0006-3002(63)90753-4. [DOI] [PubMed] [Google Scholar]


