Abstract
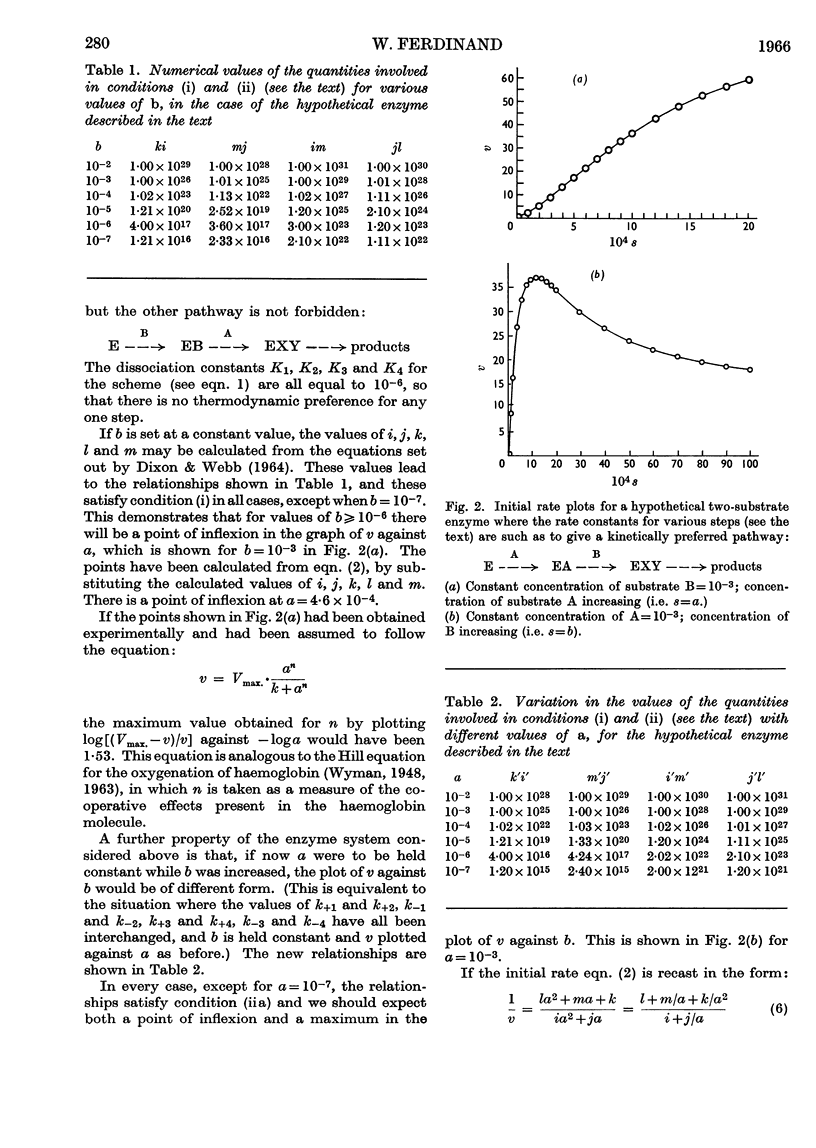
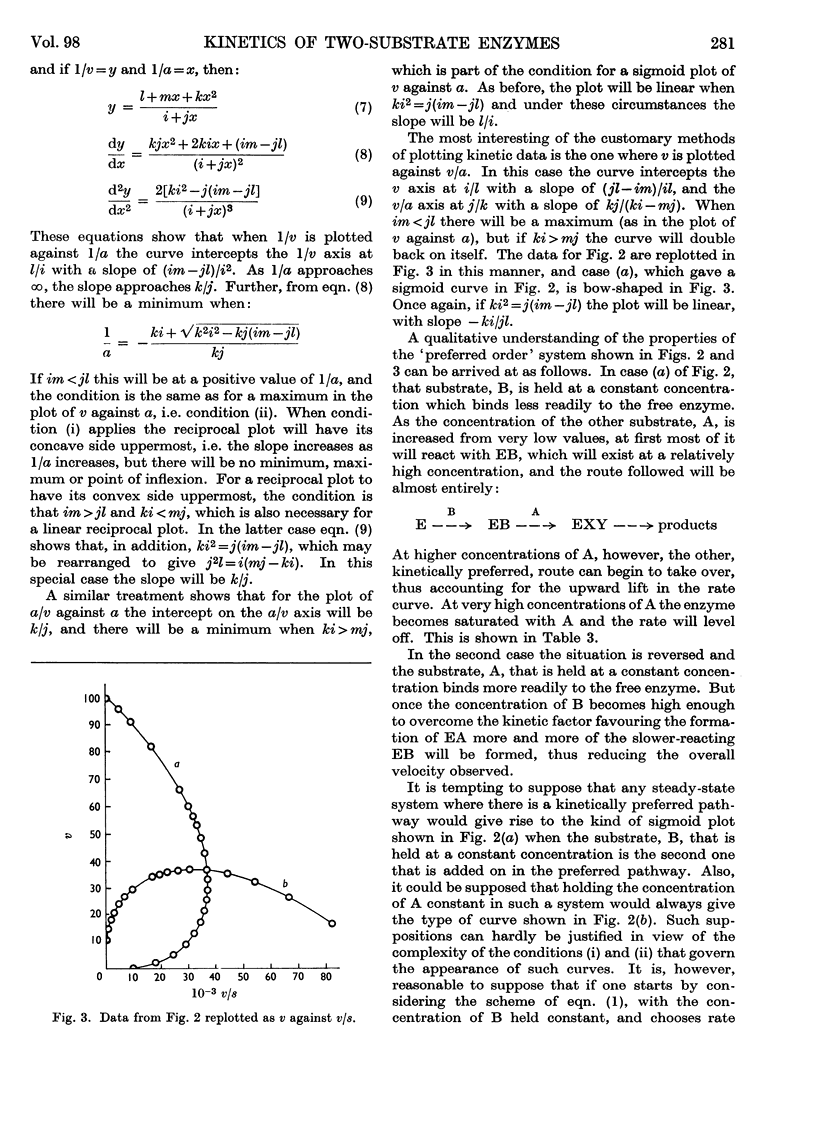
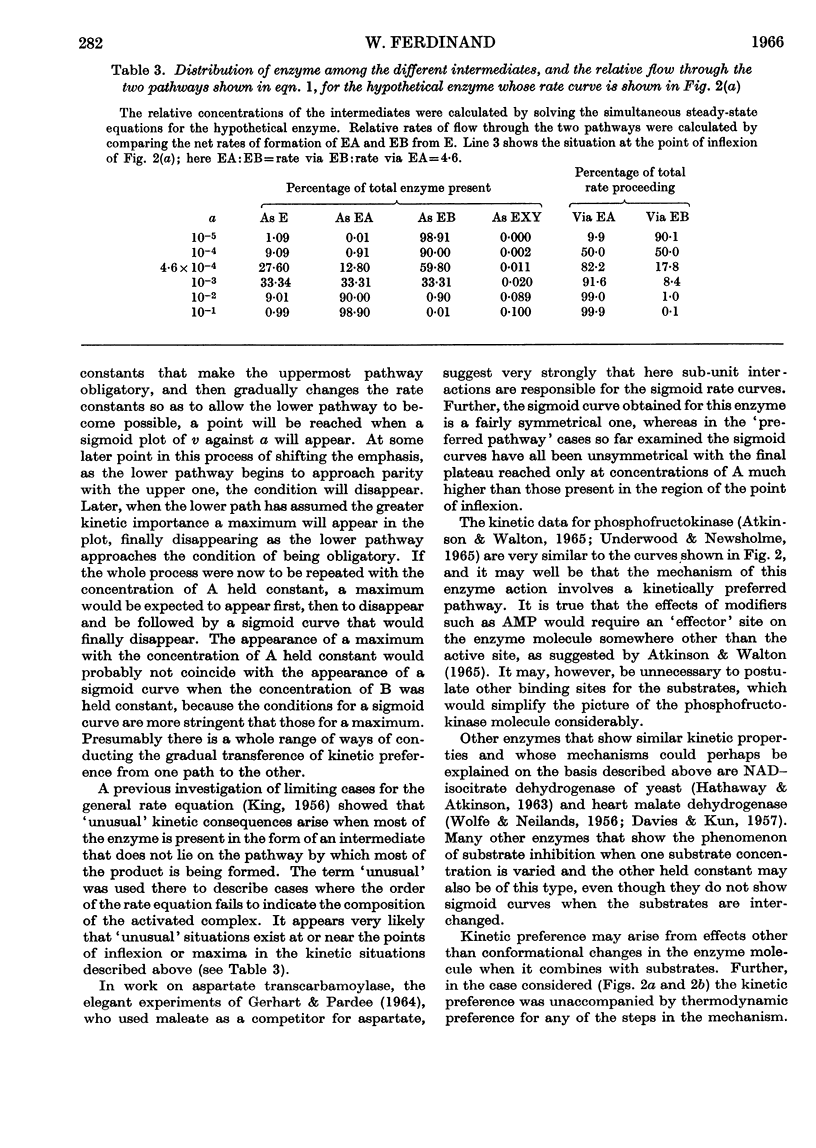
1. A theoretical appraisal of the alternative pathway mechanism for a two-substrate enzyme shows that this mechanism is capable of giving rise to apparent substrate inhibition or substrate activation (Dalziel, 1958). It has now been shown that these phenomena may occur simultaneously in the following ways. With certain relationships between the kinetic parameters and the constant concentration of one substrate, A, the plot of initial rate, v, against the concentration of the other substrate, B, may show substrate `activation' at low concentrations of B and substrate `inhibition' at high concentrations of B. In other circumstances the plot of v against [B], with [A] constant, may be sigmoid (substrate activation), whereas the plot of v against [A], with [B] constant, may pass through a maximum (substrate inhibition). 2. Kinetic data for phosphofructokinase are of the latter type and it is suggested that the mechanism of this enzyme may involve a kinetically preferred pathway. It is emphasized that the phenomena of substrate inhibition and activation need not necessarily involve more than one binding site for each substrate on the enzyme molecule, nor more than one monomer per molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTY R. A. Enzyme kinetics. Adv Enzymol Relat Subj Biochem. 1956;17:1–64. doi: 10.1002/9780470122624.ch1. [DOI] [PubMed] [Google Scholar]
- ATKINSON D. E., WALTON G. M. KINETICS OF REGULATORY ENZYMES. ESCHERICHIA COLI PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 Feb;240:757–763. [PubMed] [Google Scholar]
- DAVIES D. D., KUN E. Isolation and properties of malic dehydrogenase from ox-heart mitochondria. Biochem J. 1957 Jun;66(2):307–316. doi: 10.1042/bj0660307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. V. Kinetic studies of substrate inhibition by oxalacetate. Biochemistry. 1963 Mar-Apr;2:220–224. doi: 10.1021/bi00902a003. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE R. G., NEILANDS J. B. Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem. 1956 Jul;221(1):61–69. [PubMed] [Google Scholar]


