Abstract
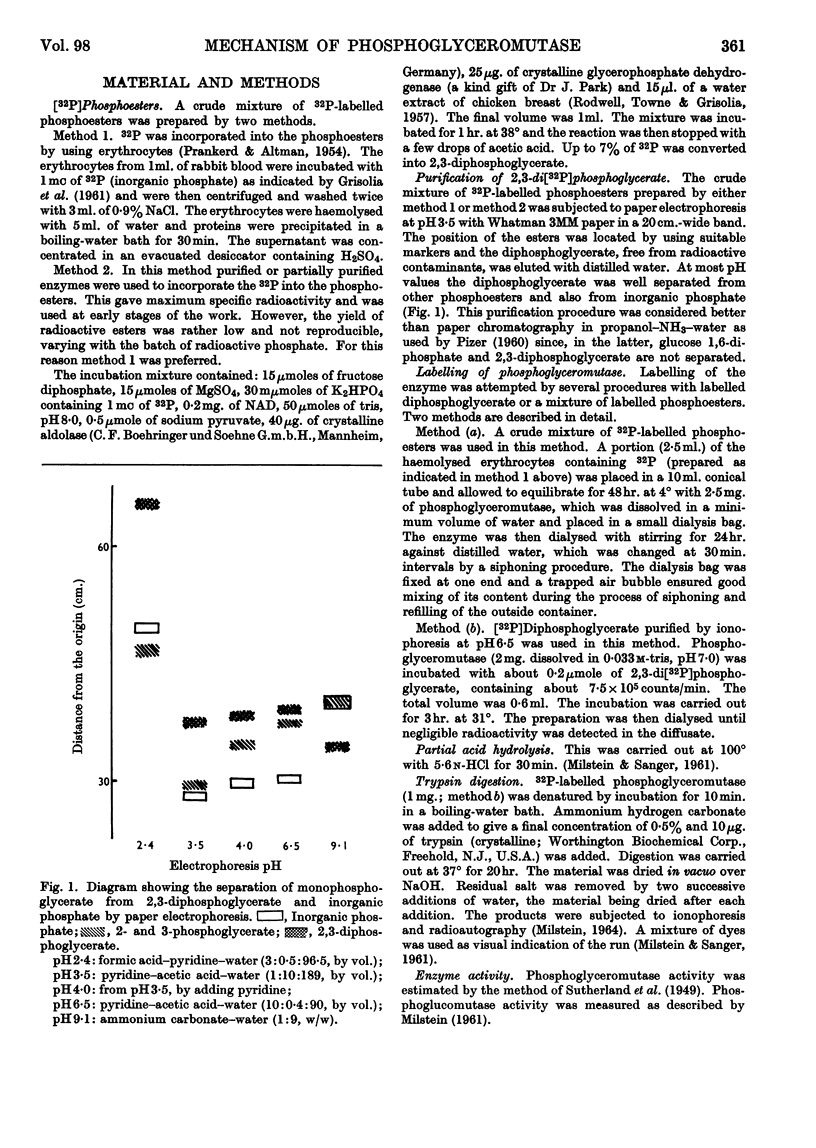
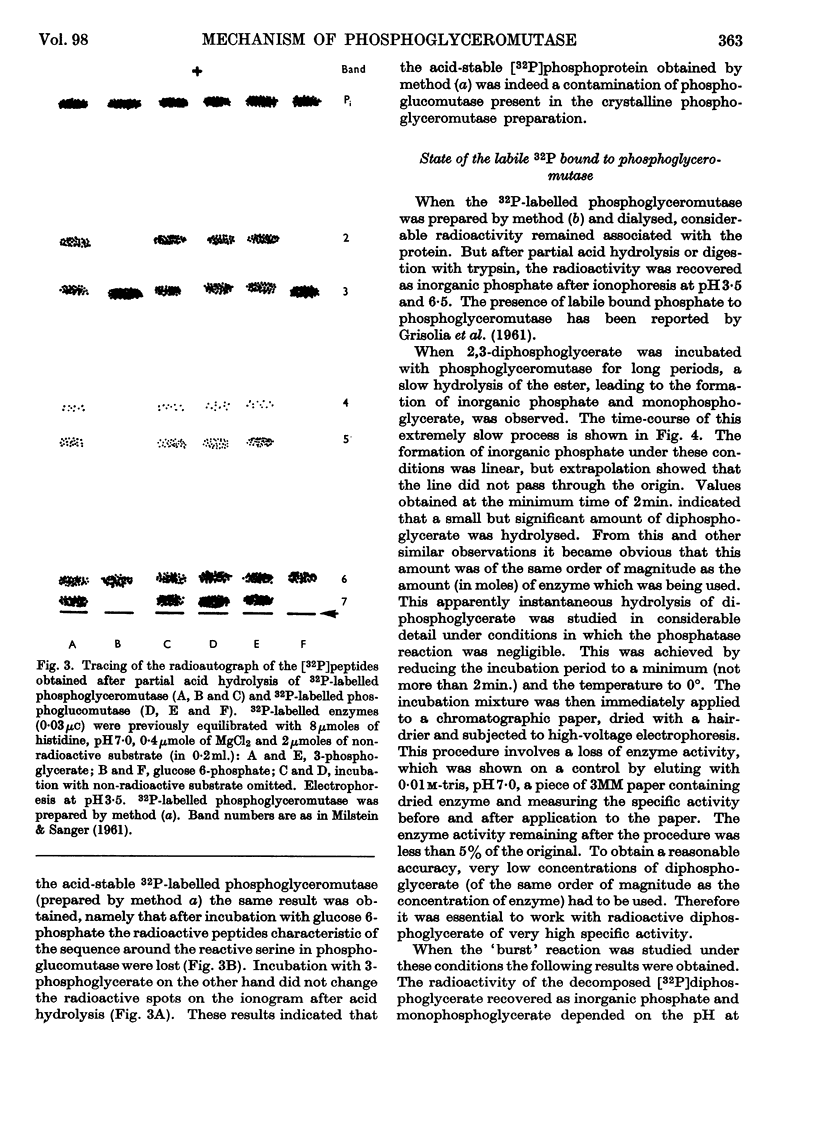
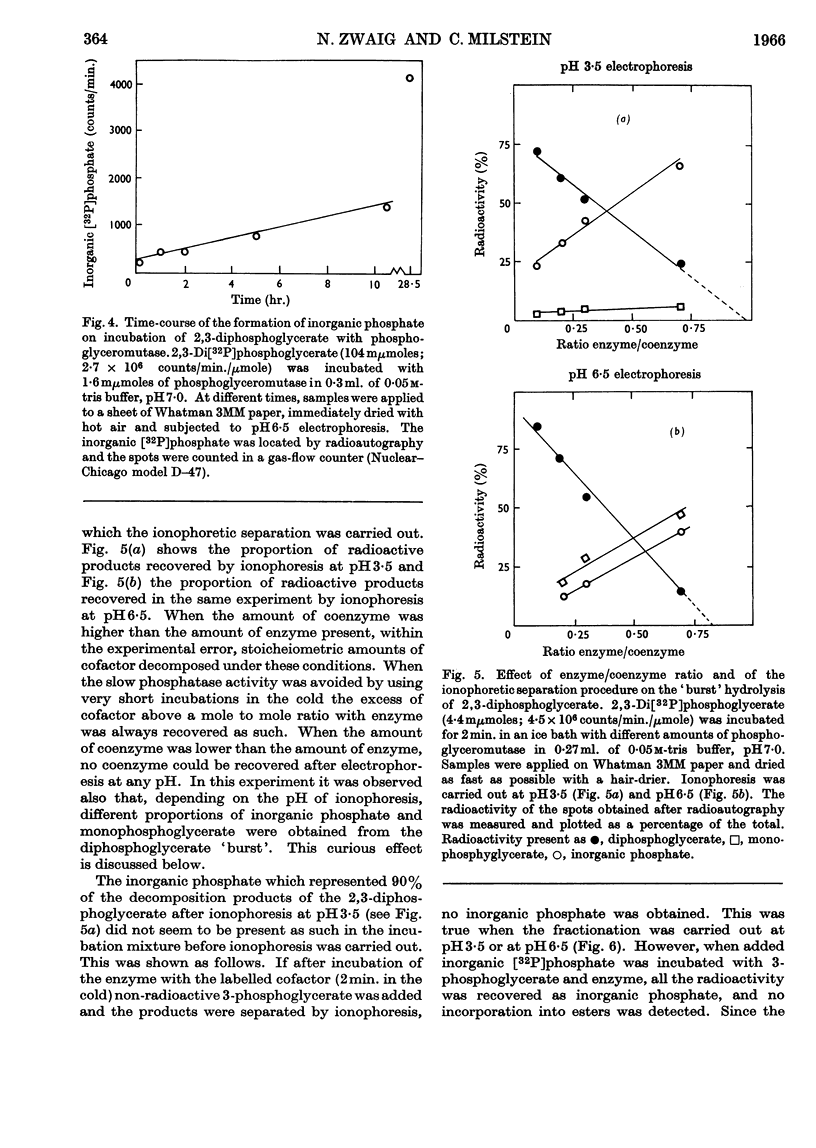
1. High-voltage paper-electrophoresis methods have been used for the separation of 32P-labelled phosphoesters. 2. Evidence is presented which indicates that 32P-labelled phosphopeptides, obtained after acid hydrolysis of phosphoglyceromutase incubated with impure 2,3-di[32P]phosphoglycerate, are derived from phosphoglucomutase contamination. 3. The hydrolysis of 2,3-di[32P]phosphoglycerate by phosphoglyceromutase has been studied. After an apparent instantaneous hydrolysis of 1 mole of coenzyme/mole of enzyme the reaction proceeds at a very low rate. 4. This hydrolysis seems to be due to the destruction of an enzyme–coenzyme complex. The proportions of the decomposition products of the complex vary according to further handling (pH of ionophoresis). 5. The inorganic [32P]phosphate produced by the hydrolysis of the complex and the inorganic [32P]phosphate produced by the slow phosphatase activity can be differentiated by the ability of the former to be incorporated into non-radioactive substrate before enzyme denaturation. 6. The effect of enzyme concentration on the stoicheiometry of the slow phosphatase hydrolysis of the diphosphoglycerate is described and this suggests that it may occur via two independent reactions, one of them being the decomposition of the enzyme–coenzyme complex on standing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON L., JOLLES G. R. A study of the linkage of phosphorus to protein in phosphoglucomutase. Arch Biochem Biophys. 1957 Jul;70(1):121–128. doi: 10.1016/0003-9861(57)90085-1. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- GRISOLIA S., JOYCE B. K., FERNANDEZ M. Studies on the mechanism of action of phosphoglyceromutases. Biochim Biophys Acta. 1961 Jun 10;50:81–89. doi: 10.1016/0006-3002(61)91063-0. [DOI] [PubMed] [Google Scholar]
- JOYCE B. K., GRISOLIA S. Studies on glycerate 2,3-diphosphatase. J Biol Chem. 1958 Aug;233(2):350–354. [PubMed] [Google Scholar]
- MILSTEIN C. On the mechanism of activation of phosphoglucomutase by metal ions. Biochem J. 1961 Jun;79:574–584. doi: 10.1042/bj0790574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAJJAR V. A., PULLMAN M. E. The occurrence of a group transfer involving enzyme (phosphoglucomutase) and substrate. Science. 1954 May 7;119(3097):631–634. doi: 10.1126/science.119.3097.631. [DOI] [PubMed] [Google Scholar]
- PIZER L. I. Properties of the phosphoprotein-phosphoglyceric mutase. J Biol Chem. 1960 Apr;235:895–901. [PubMed] [Google Scholar]
- PRANKERD T. A., ALTMAN K. I. A study of the metabolism of phosphorus in mammalian red cells. Biochem J. 1954 Dec;58(4):622–633. doi: 10.1042/bj0580622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY W. J., Jr, ROSCELLI G. A. A KINETIC STUDY OF THE PHOSPHOGLUCOMUTASE PATHWAY. J Biol Chem. 1964 Apr;239:1228–1236. [PubMed] [Google Scholar]
- RODWELL V. W., TOWNE J. C., GRISOLIA S. The kinetic properties of yeast and muscle phosphoglyceric acid mutase. J Biol Chem. 1957 Oct;228(2):875–890. [PubMed] [Google Scholar]
- SUTHERLAND E. W., POSTERNAK T., CORI C. F. Mechanism of the phosphoglyceric mutase reaction. J Biol Chem. 1949 Nov;181(1):153–159. [PubMed] [Google Scholar]
- ZWAIG N., MILSTEIN C. ON THE NATURE OF THE PHOSPHOENZYME INTERMEDIATE IN THE PHOSPHOGLYCEROMUTASE REACTION. Biochim Biophys Acta. 1963 Aug 6;73:676–679. doi: 10.1016/0006-3002(63)90347-0. [DOI] [PubMed] [Google Scholar]


