Abstract
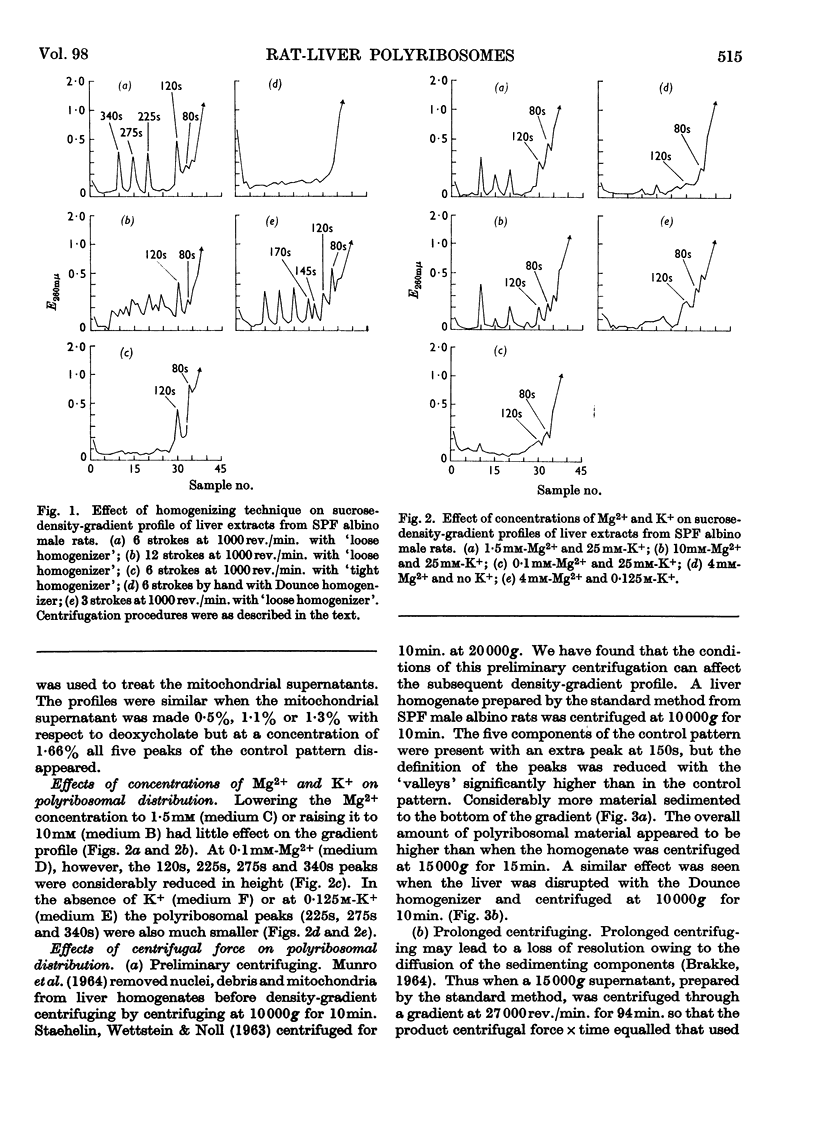
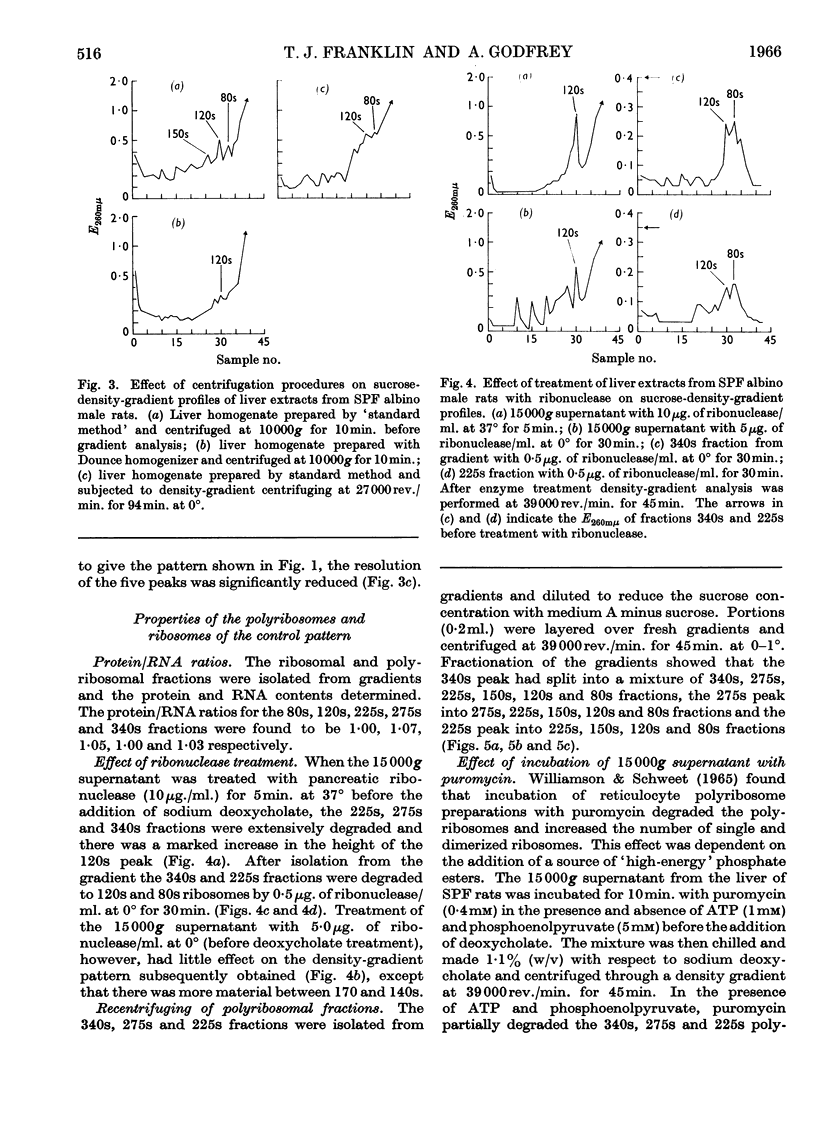
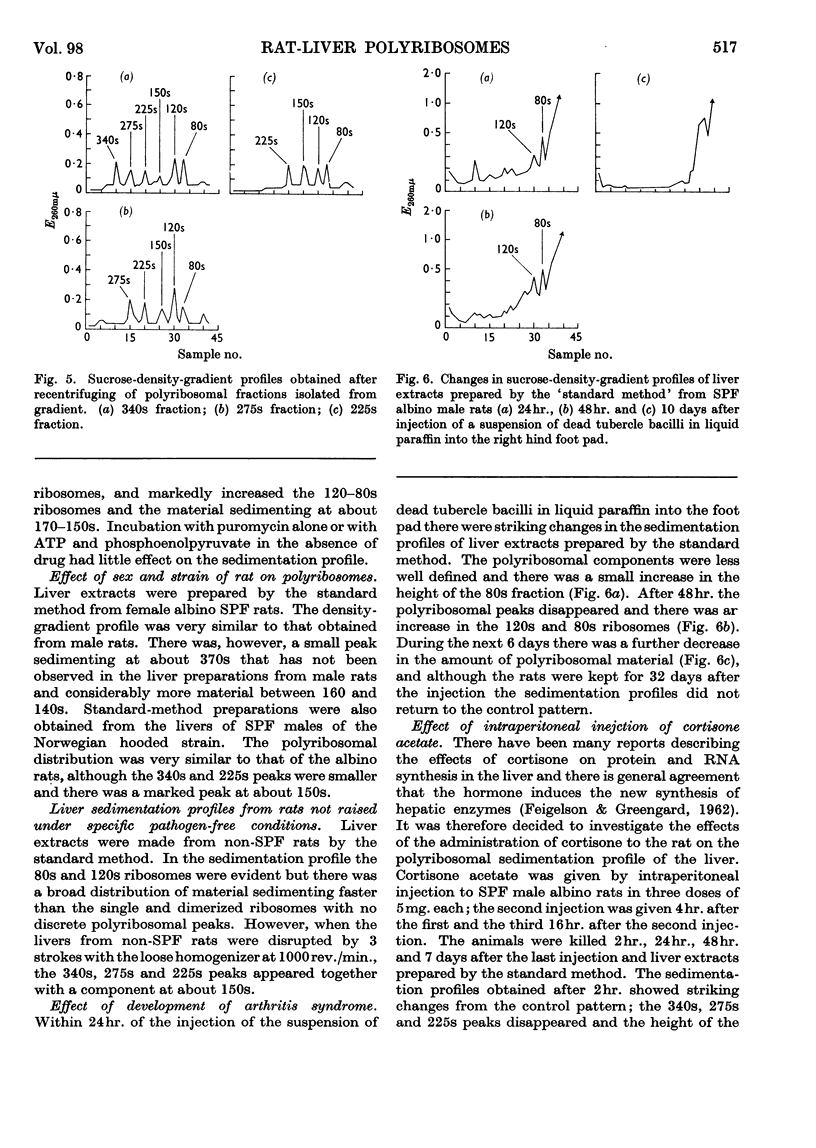
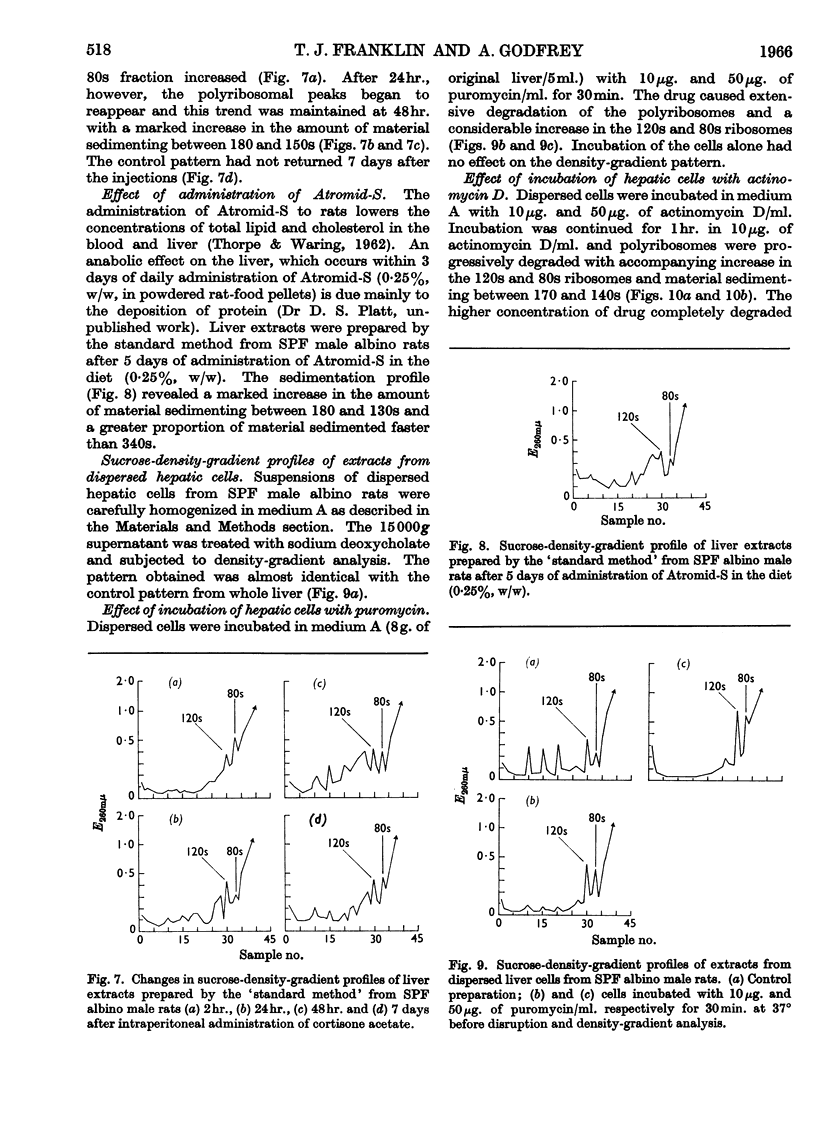
1. The distribution of rat-liver polyribosomes in sucrose density gradients has been investigated with regard to the effects of the preparative procedures and the physiological and pathological condition of the animal. 2. By using carefully defined conditions, three principal polyribosomal fractions have been isolated with S20,w values of 340, 275 and 225s in addition to the dimerized 120s and single 80s ribosomes. 3. The polyribosomes were very sensitive to treatment with ribonuclease and to mechanical stresses. 4. Incubation of dispersed hepatic cells and also cell-free preparations with puromycin in the presence of ATP and phosphoenolpyruvate caused rapid partial degradation of the polyribosomes. Treatment of the dispersed cells with actinomycin D also degraded the polyribosomes. 5. The liver polyribosomes of rats not raised under pathogen-free conditions and possibly of rats with an arthritic syndrome may be more fragile than those of healthy pathogen-free animals. 6. Treatment of pathogen-free rats with drugs stimulating liver anabolism profoundly affected the distribution of polyribosomes in sucrose density gradients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAKKE M. K. NONIDEAL SEDIMENTATION AND THE CAPACITY OF SUCROSE GRADIENT COLUMNS FOR VIRUS IN DENSITY-GRADIENT CENTRIFUGATION. Arch Biochem Biophys. 1964 Sep;107:388–403. doi: 10.1016/0003-9861(64)90295-4. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Daly J. M. Density-Gradient Centrifugation: Non-Ideal Sedimentation and the Interaction of Major and Minor Components. Science. 1965 Apr 16;148(3668):387–389. doi: 10.1126/science.148.3668.387. [DOI] [PubMed] [Google Scholar]
- LAMFROM H., KNOPF P. M. PROPERTIES OF RETICULOCYTE 80 S RIBOSOMES. J Mol Biol. 1965 Mar;11:589–599. doi: 10.1016/s0022-2836(65)80013-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWBOULD B. B. CHEMOTHERAPY OF ARTHRITIS INDUCED IN RATS BY MYCOBACTERIAL ADJUVANT. Br J Pharmacol Chemother. 1963 Aug;21:127–136. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERMANN M. L. Ribonucleoprotein from a rat tumor, the Jensen sarcoma. I. The effect of magnesium binding on ultracentrifugal and electrophoretic properties. J Biol Chem. 1960 Jul;235:1998–2003. [PubMed] [Google Scholar]
- REVEL M., HIATT H. H. THE STABILITY OF LIVER MESSENGER RNA. Proc Natl Acad Sci U S A. 1964 May;51:810–818. doi: 10.1073/pnas.51.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIFKIND R. A., LUZZATTO L., MARKS P. A. SIZE OF POLYRIBOSOMES IN INTACT RETICULOCYTES. Proc Natl Acad Sci U S A. 1964 Nov;52:1227–1232. doi: 10.1073/pnas.52.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORTMAN K. Studies on cellular inhibitors of ribonuclease. III. The levels of ribonuclease and ribonucleases inhibitor during the regeneration of rat liver. Biochim Biophys Acta. 1962 Jul 9;61:50–55. doi: 10.1016/0926-6550(62)90028-2. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., NOLL H. Breakdown of rat-liver ergosomes in vivo after actinomycin inhibition of messenger RNA synthesis. Science. 1963 Apr 12;140(3563):180–183. doi: 10.1126/science.140.3563.180. [DOI] [PubMed] [Google Scholar]
- THORP J. M., WARING W. S. Modification of metabolism and distribution of lipids by ethyl chlorophenoxyisobutyrate. Nature. 1962 Jun 9;194:948–949. doi: 10.1038/194948a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON A. R., SCHWEET R. ROLE OF THE GENETIC MESSAGE IN INITIATION AND RELEASE OF THE POLYPEPTIDE CHAIN. Nature. 1964 May 2;202:435–437. doi: 10.1038/202435a0. [DOI] [PubMed] [Google Scholar]


