Abstract
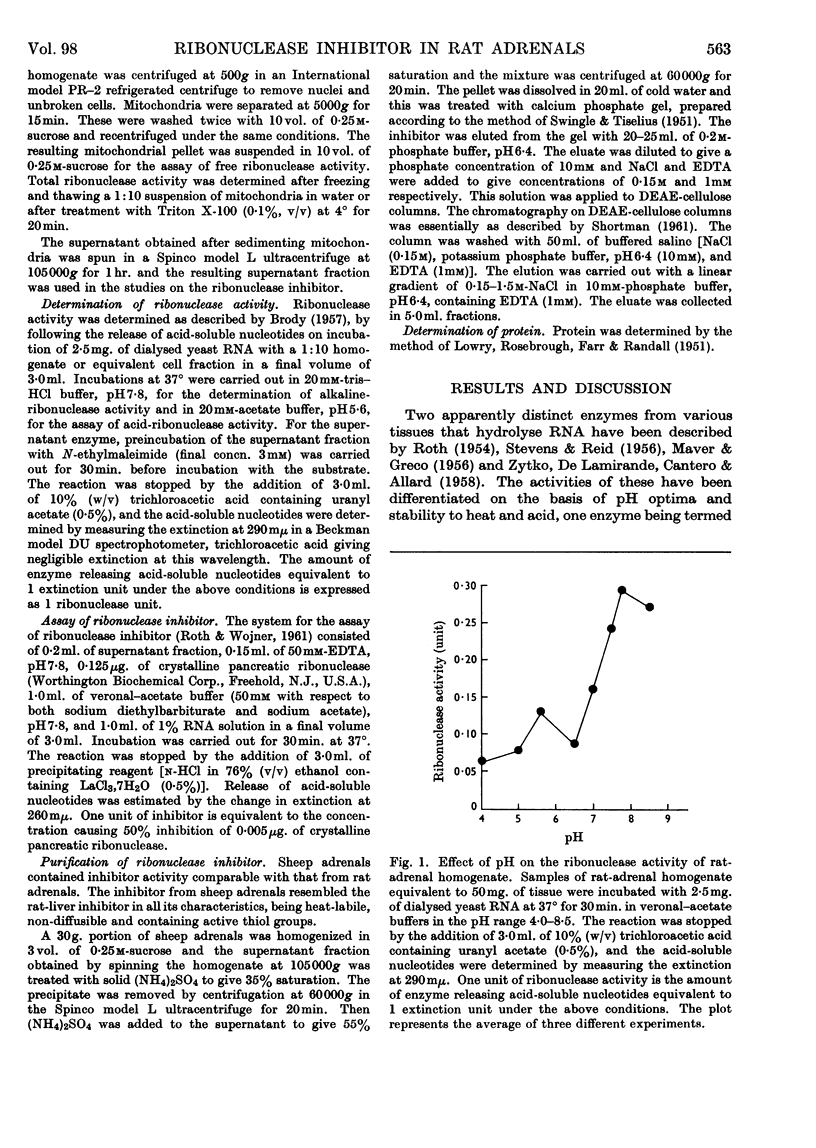
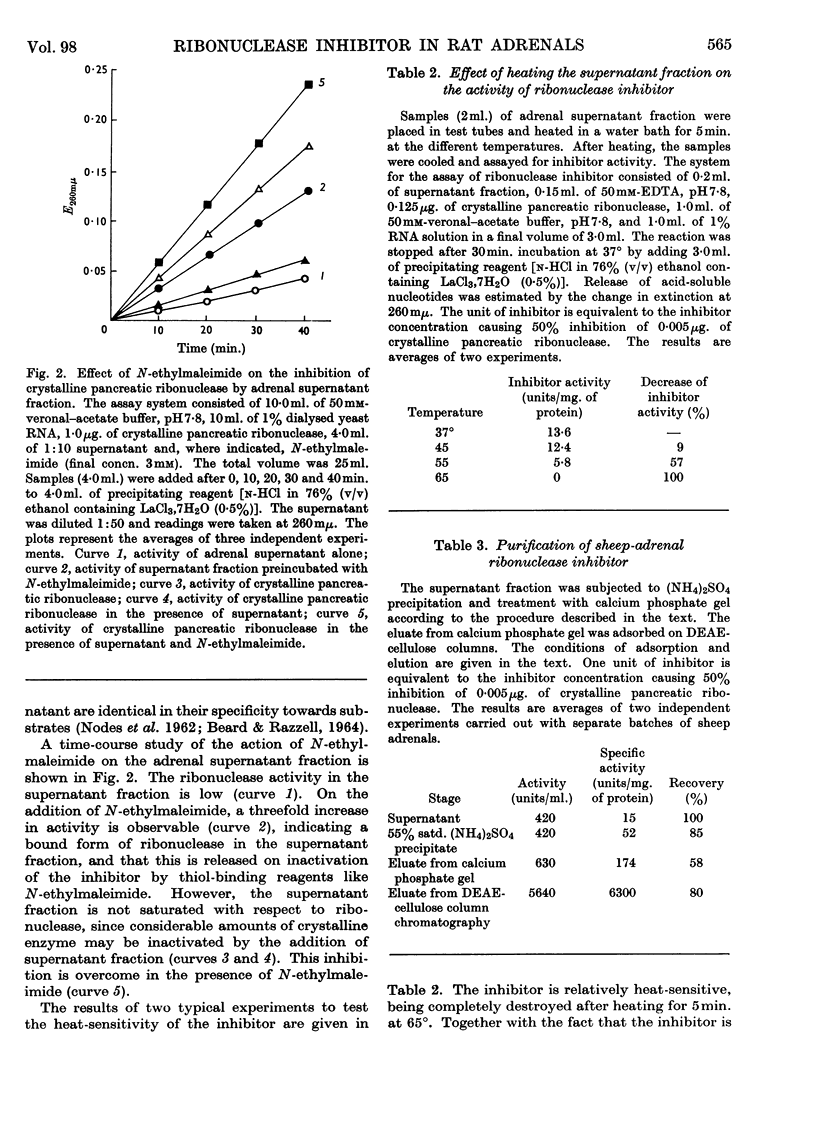
1. The presence of two RNA-degrading enzymes, one with optimum activity at pH5·6 (acid ribonuclease) and the other with optimum activity at pH7·8 (alkaline ribonuclease), in rat adrenals has been demonstrated. The acid ribonuclease was localized in the mitochondrial fraction whereas the alkaline ribonuclease was present in mitochondria as well as in the supernatant fraction. Freezing and thawing of mitochondria and treatment with Triton X-100 gave a three- to four-fold increase in acid-ribonuclease activity, whereas the mitochondrial alkaline-ribonuclease activity was practically unaffected. 2. The amount of free ribonuclease in the adrenal supernatant was small. Treatment of the supernatant fraction with N-ethylmaleimide resulted in release of large amounts of ribonuclease activity, indicating the presence of a ribonuclease inhibitor having reactive thiol groups. 3. Considerable amounts of free ribonuclease inhibitor in excess over the bound alkaline ribonuclease are present in the rat-adrenal supernatant fraction. The inhibitor is heat-labile and non-diffusible. A 400–500-fold purification of the ribonuclease inhibitor was achieved by ammonium sulphate fractionation, treatment with calcium phosphate gel and DEAE-cellulose chromatography. It is concluded that the adrenal inhibitor is protein in nature, similar to the inhibitor present in rat liver.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARTMAN M., ENGELBERG H. DEGRADATION OF RAPIDLY-TURNED-OVER RIBONUCLEIC ACID TO ACID-SOLUBLE COMPOUNDS BY ESCHERICHIA COLI RIBOSOMES. Biochim Biophys Acta. 1964 Mar 23;80:517–520. doi: 10.1016/0926-6550(64)90158-6. [DOI] [PubMed] [Google Scholar]
- BEARD J. R., RAZZELL W. E. PURIFICATION OF ALKALINE RIBONUCLEASE II FROM MITOCHONDRIAL AND SOLUBLE FRACTIONS OF LIVER. J Biol Chem. 1964 Dec;239:4186–4193. [PubMed] [Google Scholar]
- GRECO A. E., MAVER M. E. The purification and properties of deoxyribonuclease and ribonuclease from normal and neoplastic tissues. J Natl Cancer Inst. 1956 Oct;17(4):503–516. [PubMed] [Google Scholar]
- Girija N. S., Pradhan D. S., Sreenivasan A. Effect of protein depletion on ribonucleic acid metabolism in rat liver. Indian J Biochem. 1965 Jun;2(2):85–90. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAVER M. E., GRECO A. E. The chromatographic separation and characterization of the acid and alkaline ribonucleases of bovine spleen and liver. J Biol Chem. 1962 Mar;237:736–741. [PubMed] [Google Scholar]
- REID E., STEVENS B. M. Hormones and liver cytoplasm. 3. Succinic dehydrogenase, nucleases and polymerized ribonucleic acid as affected by hypophysectomy, growth-hormone treatment and adrenalectomy. Biochem J. 1956 Dec;64(4):735–740. doi: 10.1042/bj0640735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VI. Partial purification and characterization of the ribonucleases of rat liver mitochondria. J Biol Chem. 1957 Aug;227(2):591–604. [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VIII. Studies on the inactive ribonuclease in the supernatant fraction of rat liver. J Biol Chem. 1958 Apr;231(2):1097–1105. [PubMed] [Google Scholar]
- SHORTMAN K. Studies on cellular inhibitors of ribonuclease. II. Some properties of the inhibitor from rat liver. Biochim Biophys Acta. 1962 Jan 22;55:88–96. doi: 10.1016/0006-3002(62)90934-4. [DOI] [PubMed] [Google Scholar]
- SHORTMAN K. Studies on cellular inhibitors of ribonuclease. III. The levels of ribonuclease and ribonucleases inhibitor during the regeneration of rat liver. Biochim Biophys Acta. 1962 Jul 9;61:50–55. doi: 10.1016/0926-6550(62)90028-2. [DOI] [PubMed] [Google Scholar]
- SWINGLE S. M., TISELIUS A. Tricalcium phosphate as an adsorbent in the chromatography of proteins. Biochem J. 1951 Feb;48(2):171–174. doi: 10.1042/bj0480171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYTKO J., DE LAMIRANDE G., ALLARD C., CANTERO A. Ribonucleases of rat liver. I. Partial purification and properties. Biochim Biophys Acta. 1958 Mar;27(3):495–503. doi: 10.1016/0006-3002(58)90377-9. [DOI] [PubMed] [Google Scholar]


