Abstract
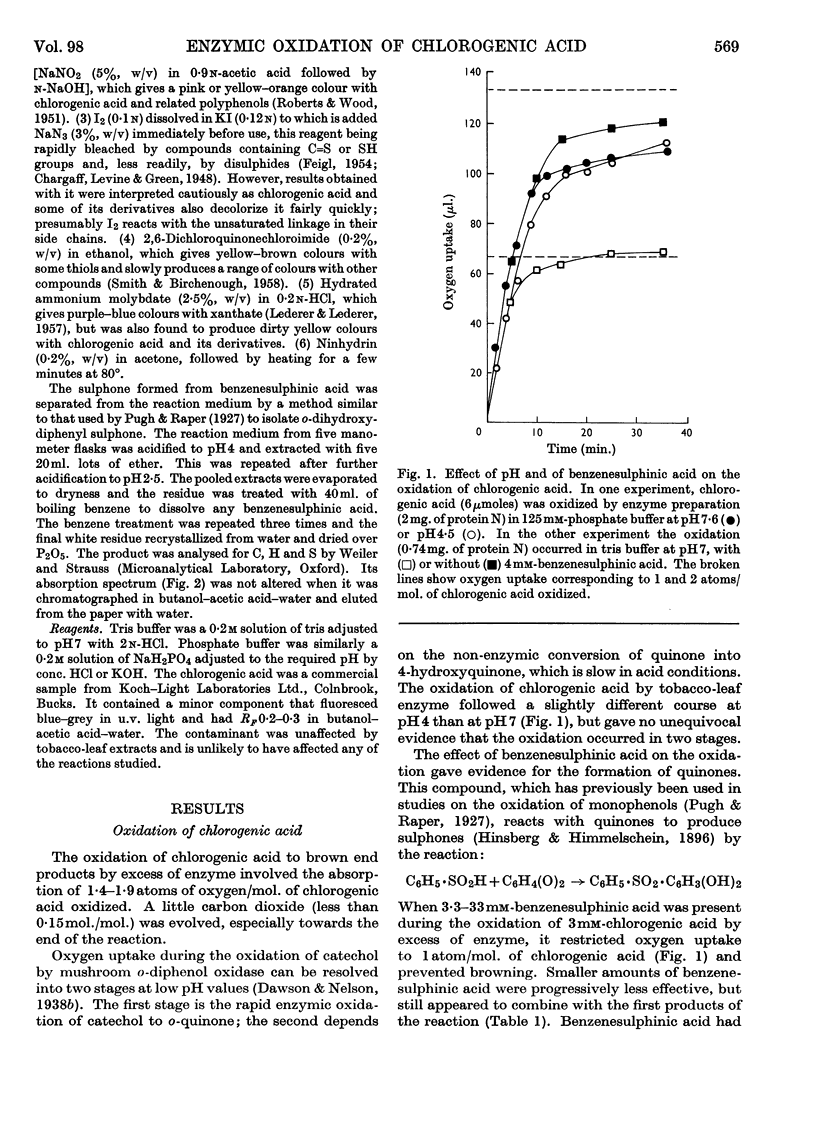
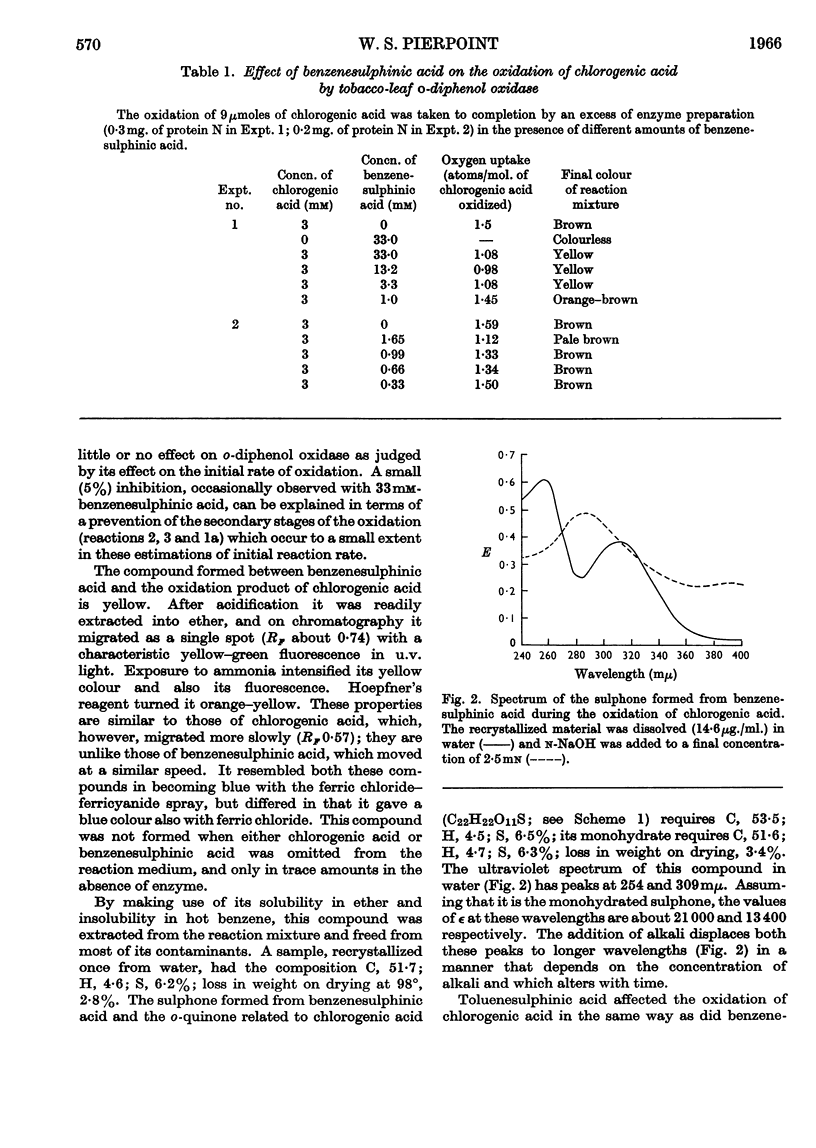
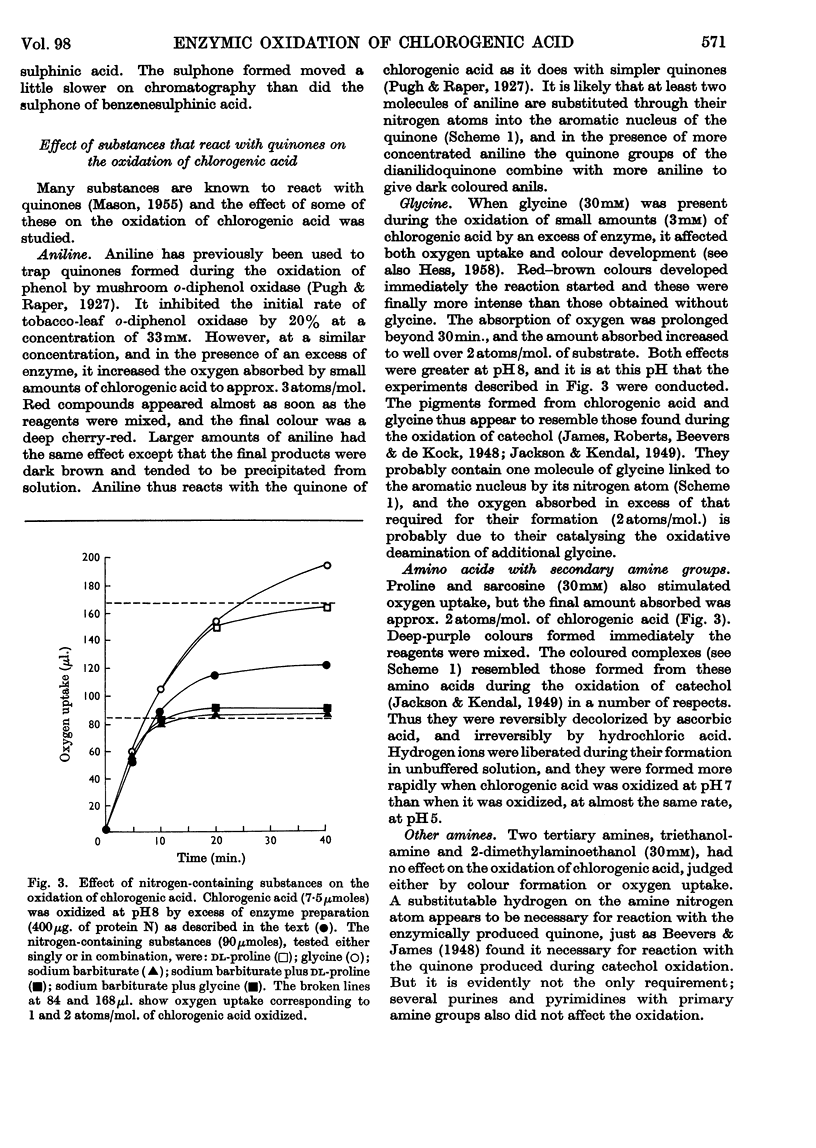
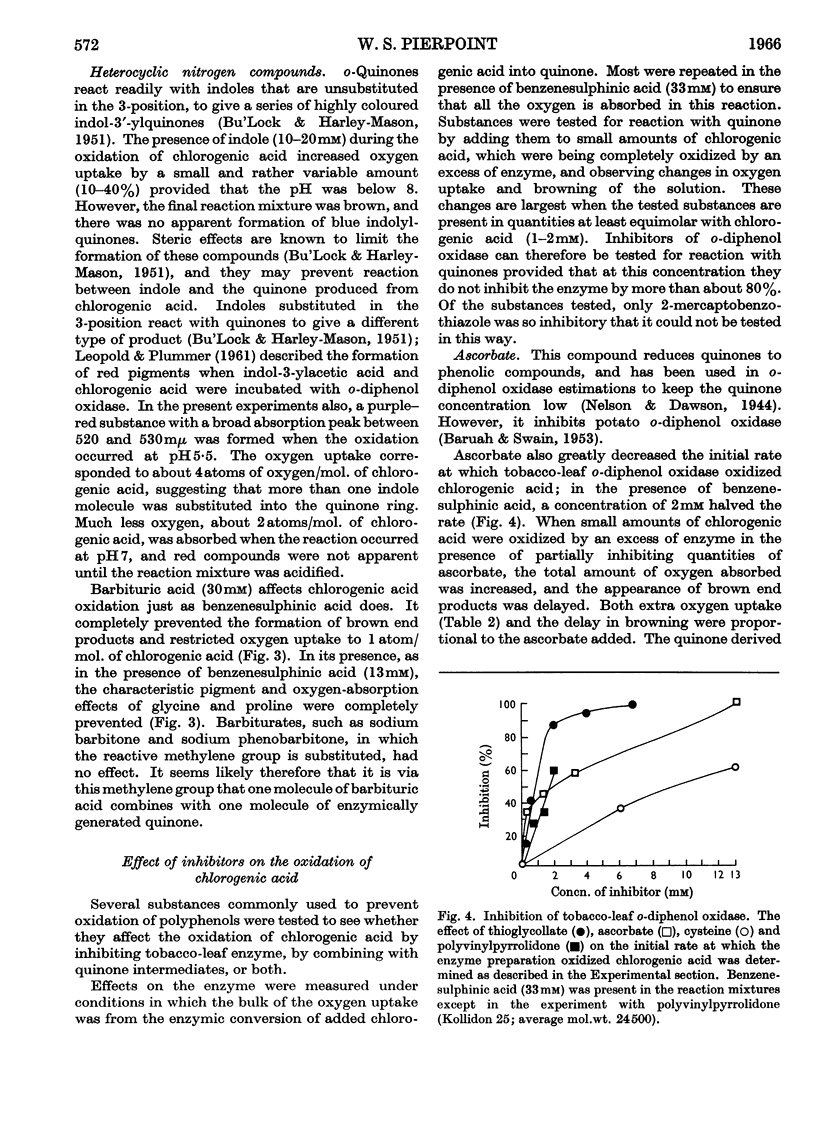
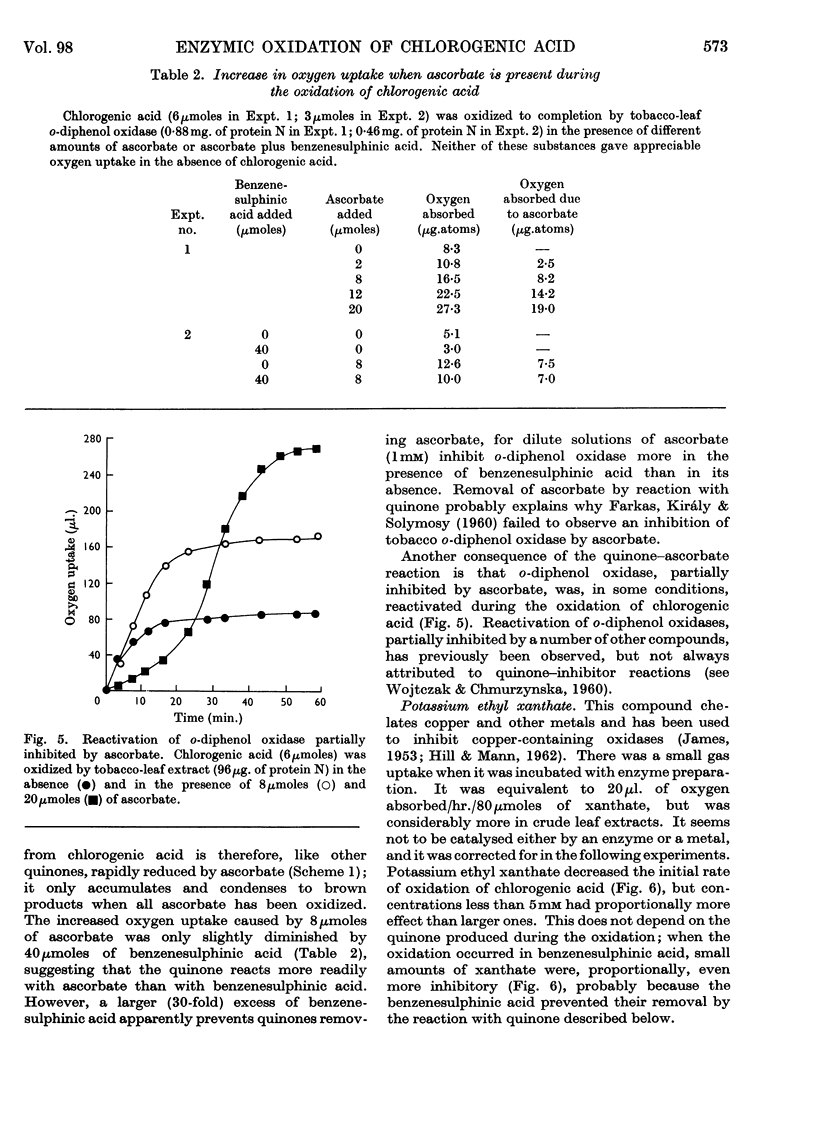
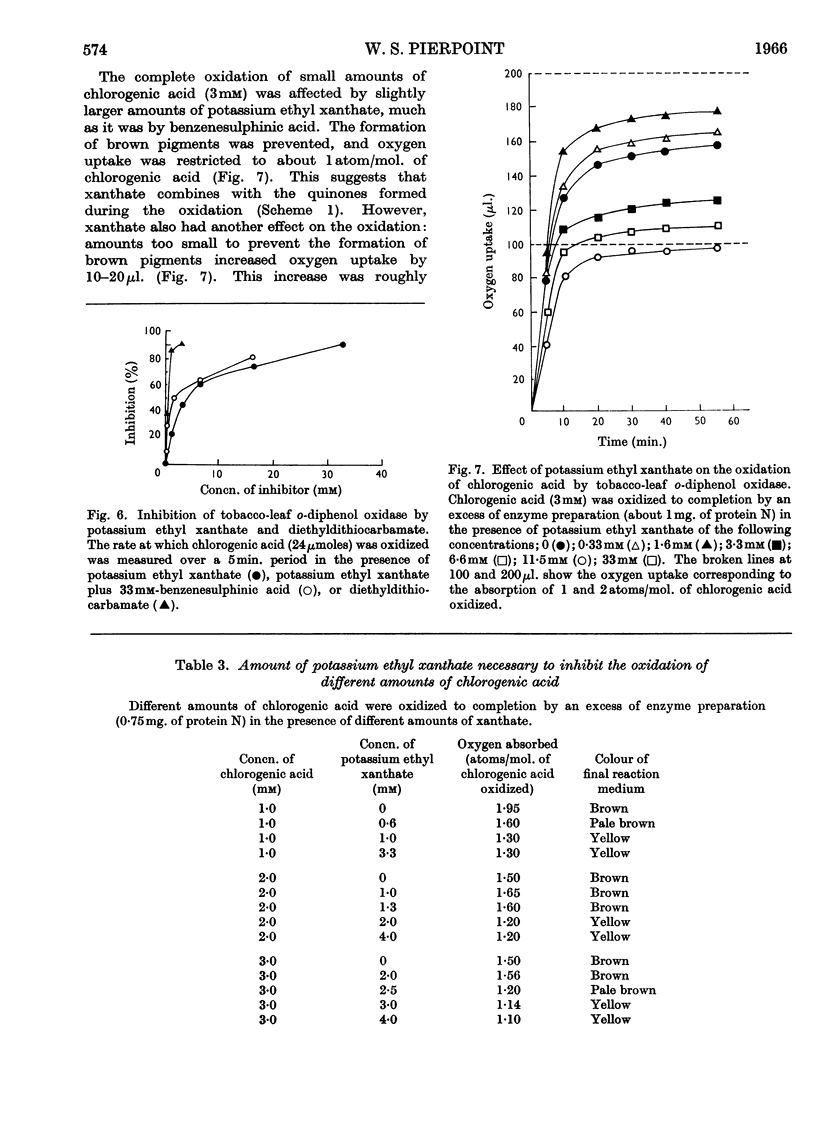
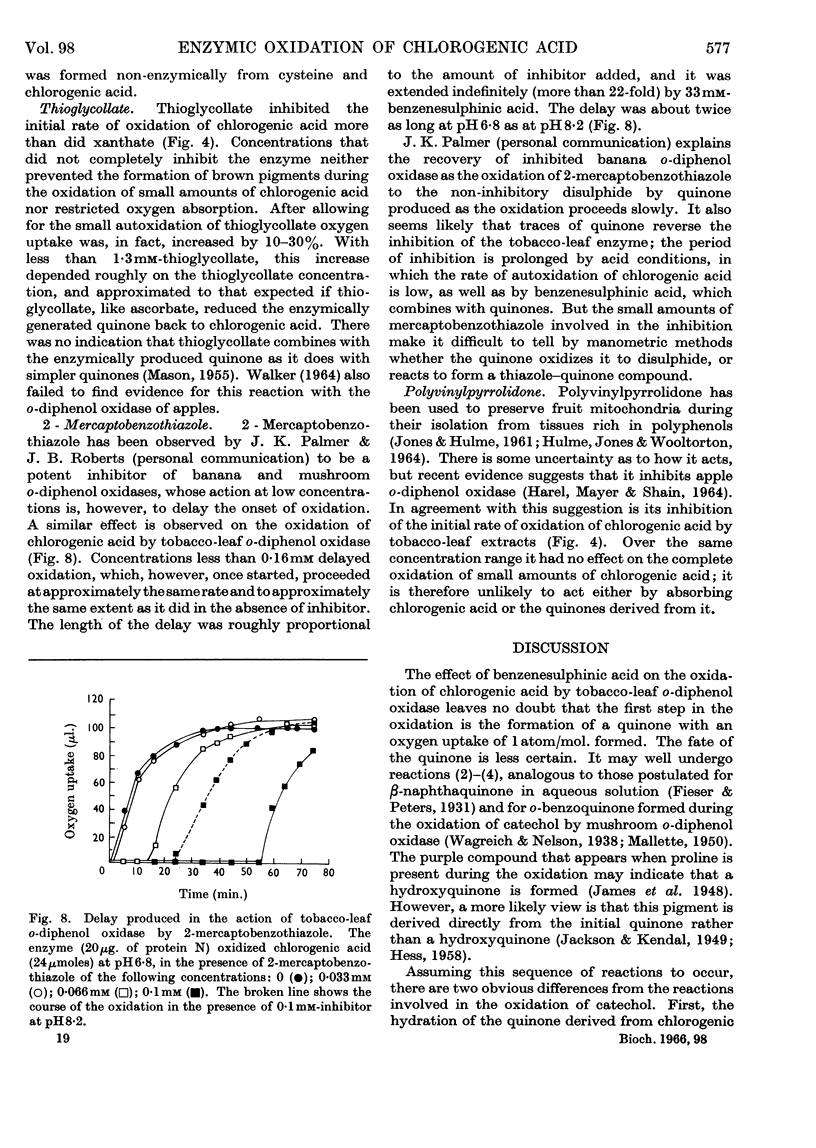
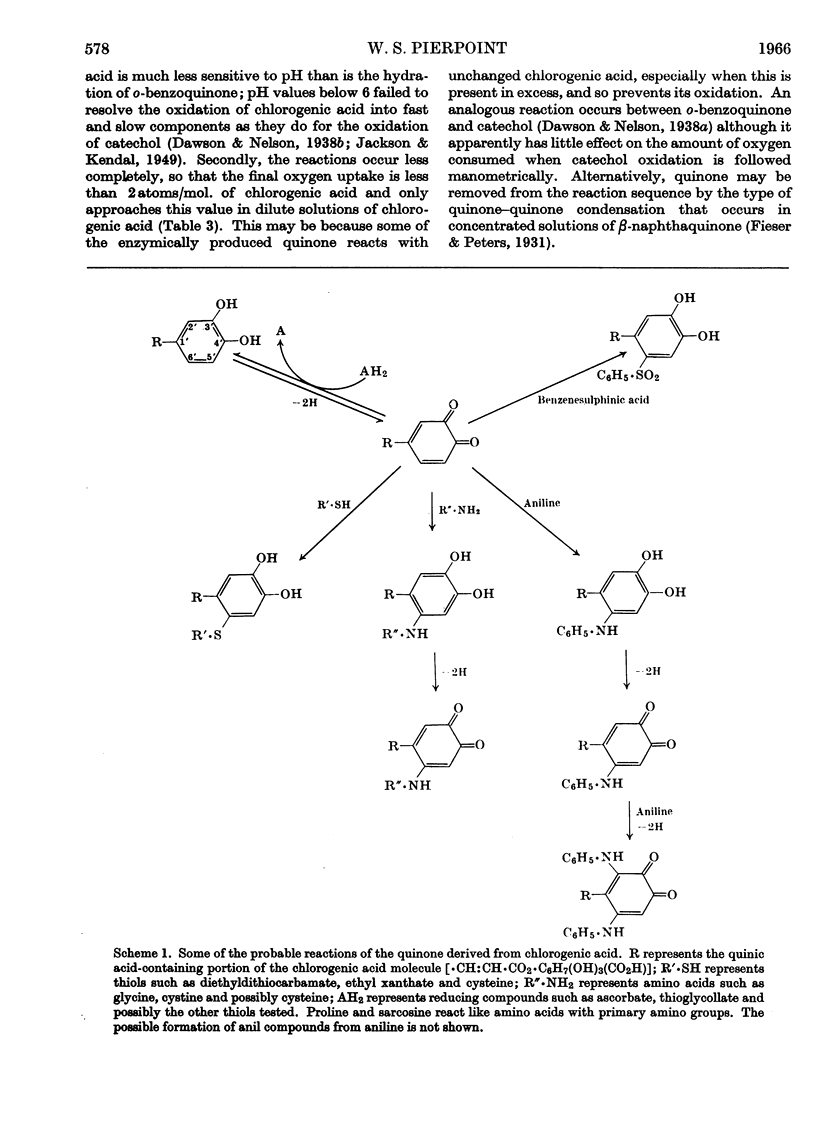
1. Partially purified preparations of tobacco-leaf o-diphenol oxidase (o-quinol–oxygen oxidoreductase; EC 1.10.3.1) oxidize chlorogenic acid to brown products, absorbing, on average, 1·6atoms of oxygen/mol. oxidized, and evolving a little carbon dioxide. 2. The effect of benzenesulphinic acid on the oxidation suggests that the first stage is the formation of a quinone; the solution does not go brown, oxygen uptake is restricted to 1 atom/mol. oxidized, and a compound is produced whose composition corresponds to that of a sulphone of the quinone derived from chlorogenic acid. 3. Several other compounds that react with quinones affect the oxidation of chlorogenic acid. The colour of the products formed and the oxygen absorbed in their formation suggest that the quinone formed in the oxidation reacts with these compounds in the same way as do simpler quinones. 4. Some compounds that are often used to prevent the oxidation of polyphenols were tested to see if they act by inhibiting o-diphenol oxidase, by reacting with quinone intermediates, or both. 5. Ascorbate inhibits the enzyme and also reduces the quinone. 6. Potassium ethyl xanthate, diethyldithiocarbamate and cysteine inhibit the enzyme to different extents, and also react with the quinone. The nature of the reaction depends on the relative concentrations of inhibitor and chlorogenic acid. Excess of inhibitor prevents the solution from turning brown and restricts oxygen uptake to 1 atom/mol. of chlorogenic acid oxidized; smaller amounts do not prevent browning and slightly increase oxygen uptake. 7. 2-Mercaptobenzothiazole inhibits the enzyme, and also probably reacts with the quinone; inhibited enzyme is reactivated as if the inhibitor is removed as traces of quinone are produced. 8. Thioglycollate and polyvinylpyrrolidone inhibit the enzyme. Thioglycollate probably reduces the quinone to a small extent.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARUAH P., SWAIN T. The effect of L-ascorbic acid on the in vitro activity of polyphenoloxidase from potato. Biochem J. 1953 Oct;55(3):392–399. doi: 10.1042/bj0550392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers H., James W. O. The behaviour of secondary and tertiary amines in the presence of catechol and belladonna catechol oxidase. Biochem J. 1948;43(4):636–639. doi: 10.1042/bj0430636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. A. Properties of tobacco polyphenol oxidase. Arch Biochem Biophys. 1959 Apr;81(2):404–417. doi: 10.1016/0003-9861(59)90219-x. [DOI] [PubMed] [Google Scholar]
- FARKAS G. L., KIRALY Z., SOLYMOSY F. Role of oxidative metabolism in the localization of plant viruses. Virology. 1960 Nov;12:408–421. doi: 10.1016/0042-6822(60)90163-x. [DOI] [PubMed] [Google Scholar]
- HARRISON B. D., PIERPOINT W. S. THE RELATION OF POLYPHENOLOXIDASE IN LEAF EXTRACTS TO THE INSTABILITY OF CUCUMBER MOSAIC AND OTHER PLANT VIRUSES. J Gen Microbiol. 1963 Sep;32:417–427. doi: 10.1099/00221287-32-3-417. [DOI] [PubMed] [Google Scholar]
- HENZE R. E. Inhibition of enzymatic browning of chlorogenic acid solutions with cysteine and glutathione. Science. 1956 Jun 29;123(3209):1174–1175. doi: 10.1126/science.123.3209.1174. [DOI] [PubMed] [Google Scholar]
- HESS E. H. The polyphenolase of tobacco and its participation in amino acid metabolism. I. Manometric studies. Arch Biochem Biophys. 1958 Mar;74(1):198–208. doi: 10.1016/0003-9861(58)90213-3. [DOI] [PubMed] [Google Scholar]
- HILL J. M., MANN P. J. The inhibition of pea-seedling diamine oxidase by chelating agents. Biochem J. 1962 Oct;85:198–207. doi: 10.1042/bj0850198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULME A. C., JONES J. D., WOOLTORTON L. S. MITOCHONDRIAL PREPARATIONS FROM FLOWERS. Nature. 1964 Feb 22;201:795–797. doi: 10.1038/201795a0. [DOI] [PubMed] [Google Scholar]
- Jackson H., Kendal L. P. The oxidation of catechol and homocatechol by tyrosinase in the presence of amino-acids. Biochem J. 1949;44(4):477–487. doi: 10.1042/bj0440477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. O., Roberts E. A., Beevers H., De Kock P. C. The secondary oxidation of amino-acids by the catechol oxidase of belladonna. Biochem J. 1948;43(4):626–636. doi: 10.1042/bj0430626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERPOINT W. S., HARRISON B. D. COPPER-DEPENDENT AND IRON-DEPENDENT INACTIVATIONS OF CUCUMBER MOSAIC VIRUS BY POLYPHENOLS. J Gen Microbiol. 1963 Sep;32:429–440. doi: 10.1099/00221287-32-3-429. [DOI] [PubMed] [Google Scholar]
- Pugh C. E., Raper H. S. The Action of Tyrosinase on Phenols: With some Observations on the Classification of Oxidases. Biochem J. 1927;21(6):1370–1383. doi: 10.1042/bj0211370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOJTCZAK L., CHMURZYNSKA W. Inhibition studies on insect polyphenol oxidase. Acta Biochim Pol. 1960;7:39–49. [PubMed] [Google Scholar]


