Abstract
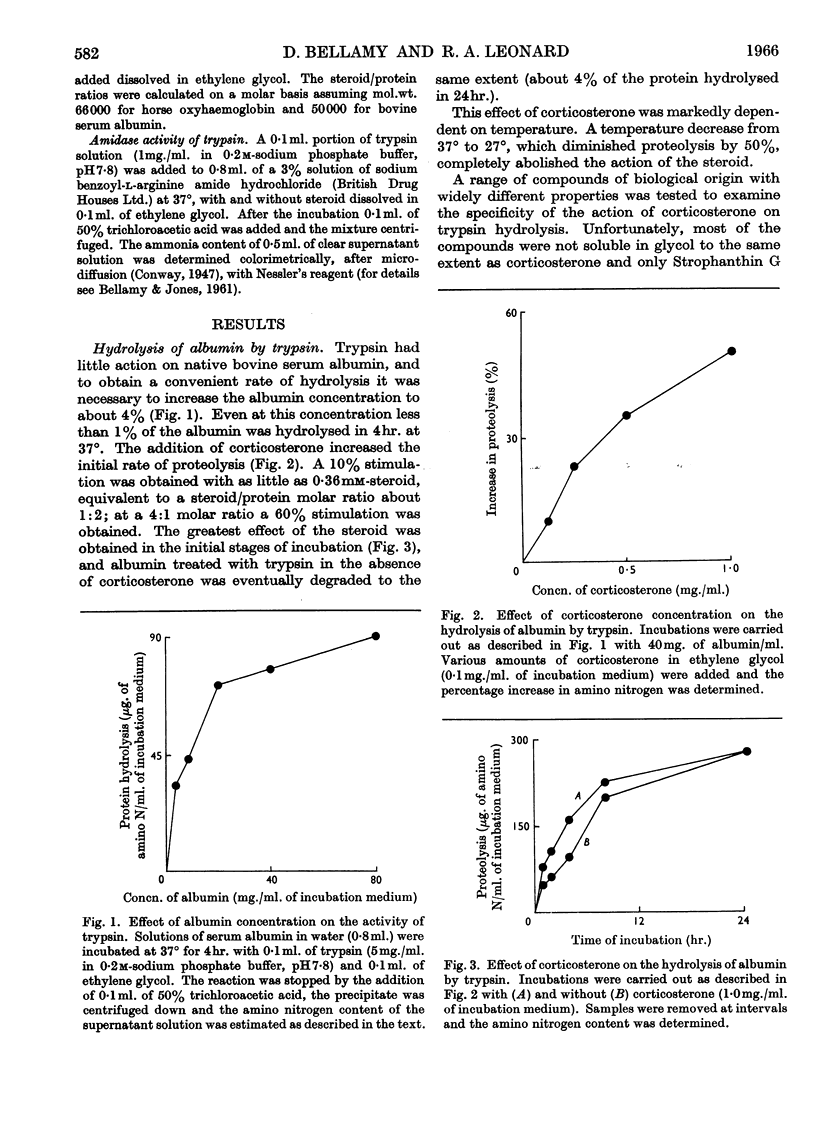
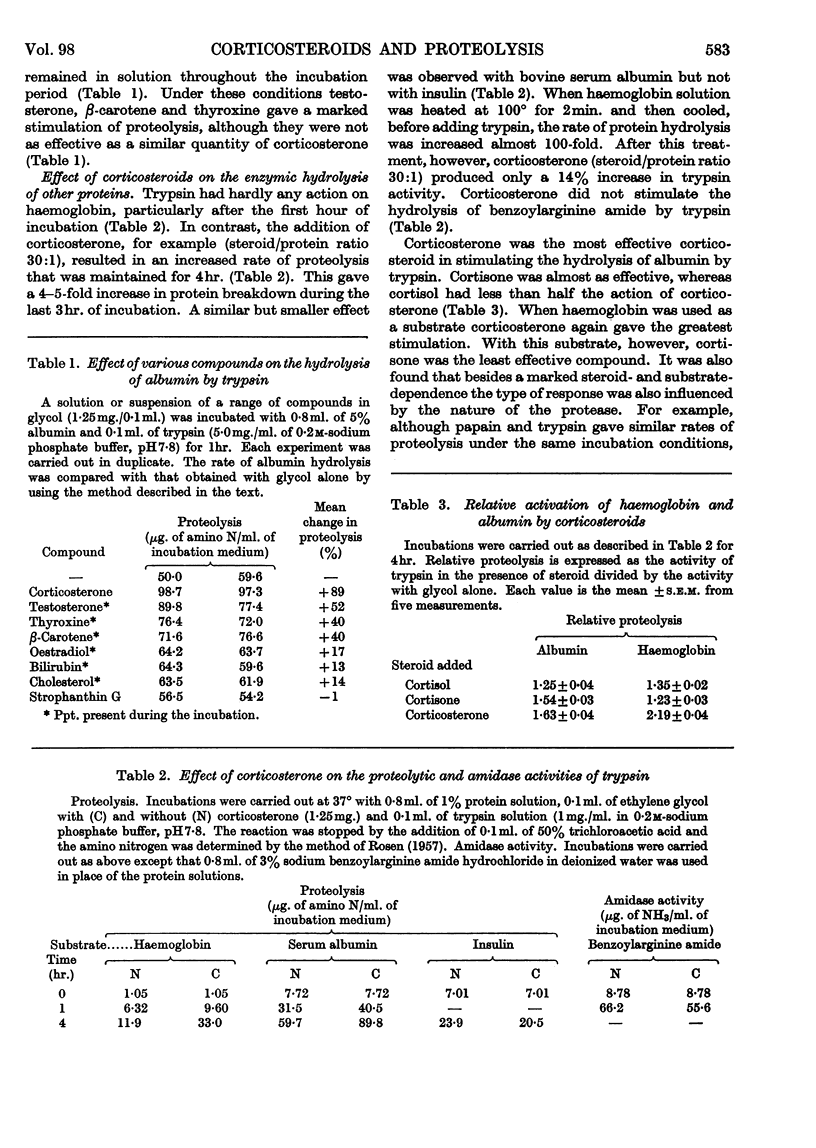
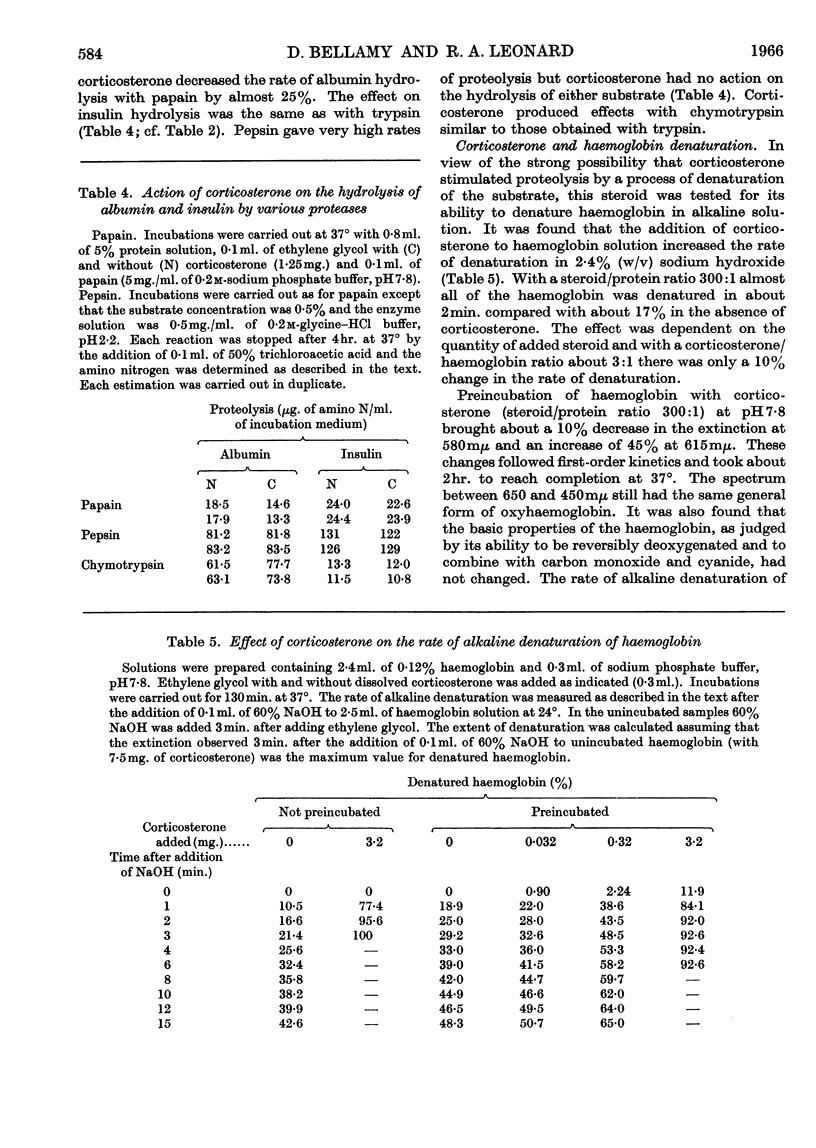
1. The corticosteroids cortisol, cortisone and corticosterone were tested for their ability to affect the hydrolysis of serum albumin, insulin and oxyhaemoglobin incubated with trypsin, chymotrypsin, papain and pepsin. 2. Corticosteroids stimulated the hydrolysis of albumin and oxyhaemoglobin with trypsin between 10% and 200% and inhibited the hydrolysis of insulin by 15% (steroid/substrate molar ratio, 5:1). 3. The degree of stimulation of proteolysis for a given substrate depended on both the nature of the steroid and the protease. Corticosterone did not increase the activity of papain and pepsin with any of the substrates tested. 4. Corticosterone stimulated (fivefold) the denaturation of oxyhaemoglobin measured spectroscopically in 2·4% (w/v) sodium hydroxide. Small changes in the absorption spectrum of haemoglobin solutions were also noted at pH7·8 without a marked change in the basic properties of haemoglobin. 5. With regard to the action of corticosterone on the activity of trypsin, the lack of stimulation when benzoylarginine amide was used as a substrate, the lowering of the stimulation on prior heat denaturation of haemoglobin and the high temperature coefficient for stimulation suggest that the steroid resulted in improved access of the protease to susceptible bonds of the substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLAMY D., JONES I. C. Studies on Myxine glutinosa. I. The chemical composition of the tissues. Comp Biochem Physiol. 1961 Oct;3:175–183. doi: 10.1016/0010-406x(61)90053-6. [DOI] [PubMed] [Google Scholar]
- BITENSKY M. W., YIELDING K. L., TOMKINS G. M. THE EFFECT OF ALLOSTERIC MODIFIERS ON THE RATE OF DENATURATION OF GLUTAMATE DEHYDROGENASE. J Biol Chem. 1965 Mar;240:1077–1082. [PubMed] [Google Scholar]
- Bellamy D., Leonard R. A. The effect of cortisol on the activity of glutamate-pyruvate transaminase and the formation of glycogen and urea in starved rats. Biochem J. 1964 Nov;93(2):331–336. doi: 10.1042/bj0930331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy D., Phillips J. G., Jones I. C., Leonard R. A. The uptake of cortisol by rat tissues. Biochem J. 1962 Dec;85(3):537–545. doi: 10.1042/bj0850537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy D. The adsorption of corticosteroids to particulate preparations of rat liver. Biochem J. 1963 May;87(2):334–340. doi: 10.1042/bj0870334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman R., Wildschut A., Wittermans A. On the occurrence of two kinds of haemoglobin in normal human blood. J Physiol. 1934 Feb 28;80(4):377–387. doi: 10.1113/jphysiol.1934.sp003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBERMAN H. D. Endocrine regulation of amino acid protein metabolism during fasting. Yale J Biol Med. 1950 Mar;22(4):341–367. [PMC free article] [PubMed] [Google Scholar]
- Jones I. C., Bellamy D. Hormonal mechanisms in the homeostatic regulation of the vertebrate body with special reference to the adrenal cortex. Symp Soc Exp Biol. 1964;18:195–236. [PubMed] [Google Scholar]
- LIEBERMAN S., TEICH S. Recent trends in the biochemistry of the steroid hormones. Pharmacol Rev. 1953 Sep;5(3):285–380. [PubMed] [Google Scholar]
- ROSSI-FANELLI A., AZZONE G. F., MONDOVI B. Alkali denaturation of myoglobin and hemoglobin; studies on the kinetics of the reaction. Arch Biochem Biophys. 1955 Sep;58(1):119–123. doi: 10.1016/0003-9861(55)90099-0. [DOI] [PubMed] [Google Scholar]
- RUSSELL J. A. Metabolic functions of the endocrine glands. Annu Rev Physiol. 1951;13:327–366. doi: 10.1146/annurev.ph.13.030151.001551. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., MAXWELL E. S. SOME ASPECTS OF STEROID HORMONE ACTION. Annu Rev Biochem. 1963;32:677–708. doi: 10.1146/annurev.bi.32.070163.003333. [DOI] [PubMed] [Google Scholar]


