Abstract
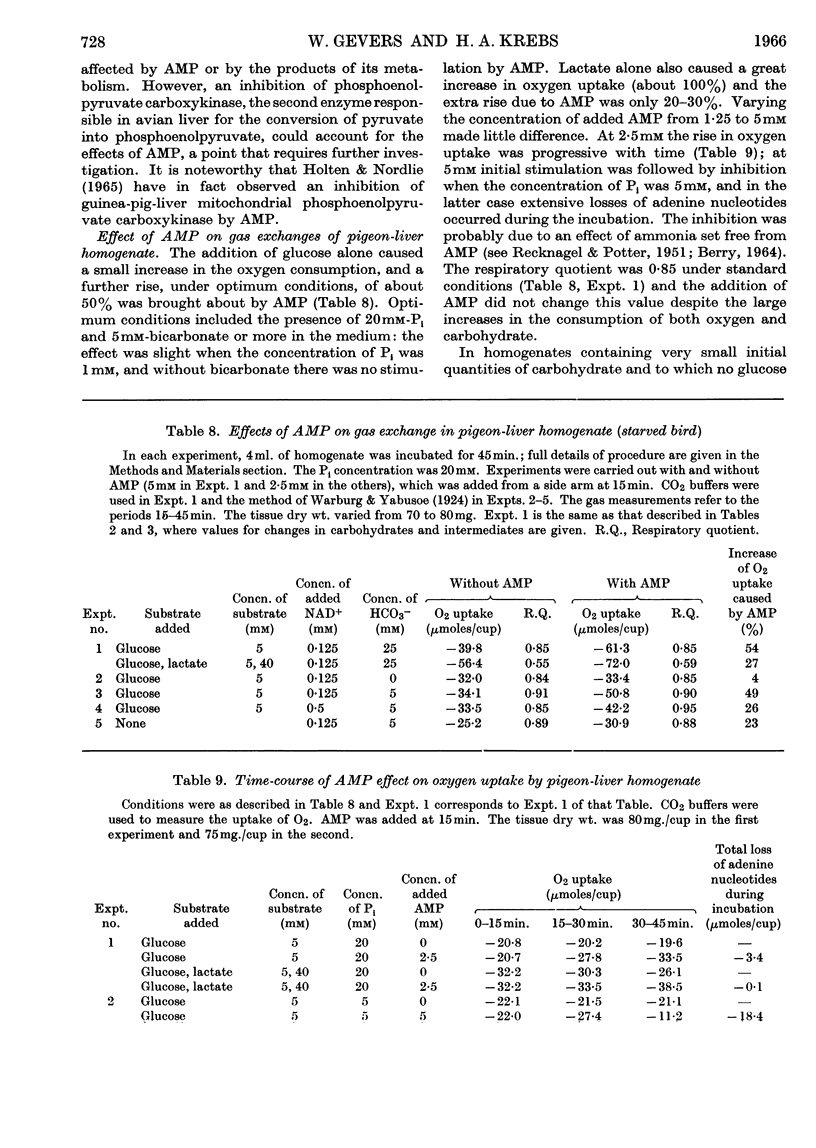
1. The effects of added AMP on carbohydrate metabolism were investigated in pigeon-liver homogenates, which can degrade glucose and synthesize it from lactate. Suitable experimental conditions were established for studying such effects, including the addition of Pi (20mm) to stabilize adenine nucleotides and supplementation with NAD+ (0·5mm). 2. Lactate increased the rate of oxygen consumption and kept the concentration of ATP high and that of AMP relatively low. 3. Added AMP (1·25–5mm) raised the net rate of carbohydrate removal and inhibited the net formation of glucose from lactate, as well as the incorporation of lactate into glucose. These effects were accompanied by a fall in the concentrations of hexose 6-phosphates and a rise in those of fructose diphosphate and triose phosphates. When the activity of glyceraldehyde 3-phosphate dehydrogenase was limited experimentally by a low concentration of NAD+ or when it was blocked by iodoacetate, the accumulations of fructose diphosphate and triose phosphates were large and accounted for most of the carbohydrate degraded in the presence of AMP. 4. AMP also inhibited the conversion of pyruvate into phosphoenolpyruvate. Data on the concentrations of pyruvate, phosphoenolpyruvate and intermediates of the tricarboxylic acid cycle, as well as on isotope distribution, suggest that the effect was due to inhibition of phosphoenolpyruvate carboxykinase. 5. The results indicate that in the homogenates phosphofructokinase and fructose diphosphatase, controlled in their activity by adenine nucleotides and other cell constituents, are enzymes which regulate the direction of carbohydrate metabolism (degradation or synthesis) in the liver. 6. It is suggested that active transport of adenine nucleotides, citrate, Mg2+, Ca2+, Pi and other cell constituents may play a role in regulating the activity of enzymes which are affected by these substances. 7. A procedure is described for generating alkali in a closed manometer vessel, by mixing mercuric oxide and a solution of sodium iodide, for use in a method for measuring the oxygen consumption at physiological bicarbonate concentrations.
Full text
PDF








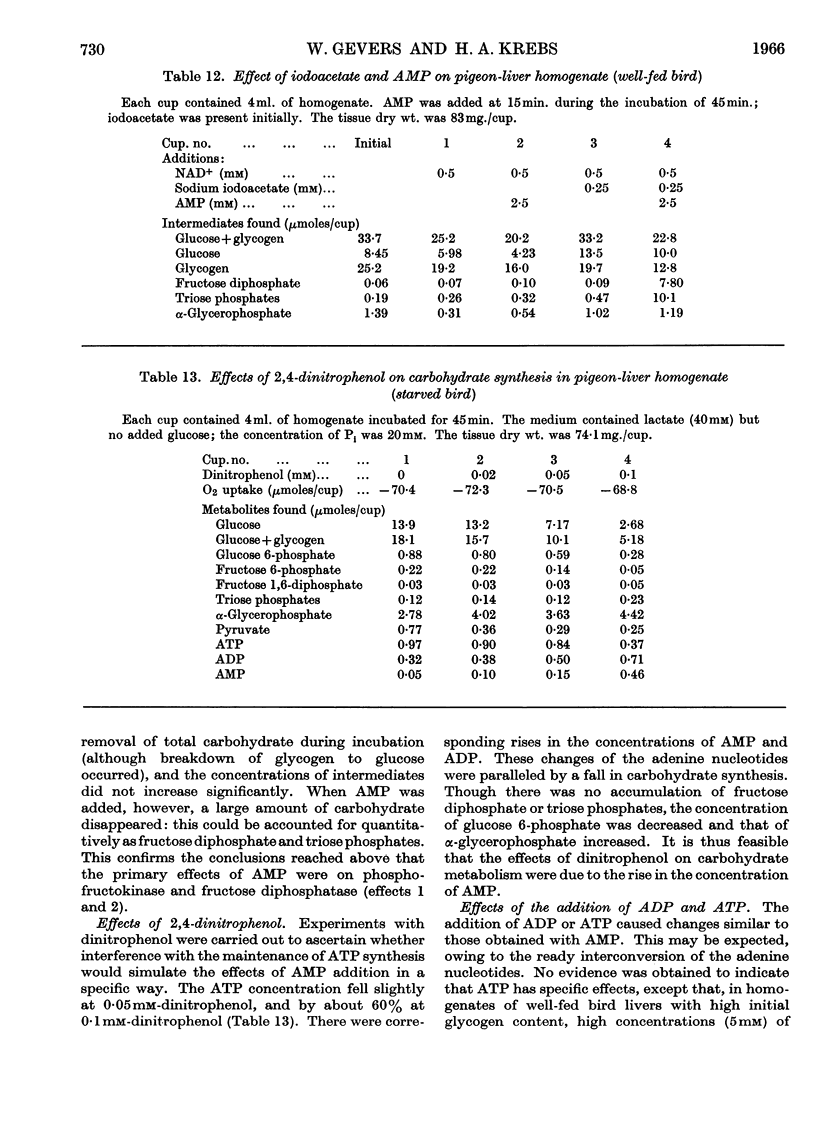




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., WALTON G. M. KINETICS OF REGULATORY ENZYMES. ESCHERICHIA COLI PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 Feb;240:757–763. [PubMed] [Google Scholar]
- BERRY M. N. THE EFFECTS OF ADENINE NUCLEOTIDES ON PYRUVATE METABOLISM IN RAT LIVER. Biochem J. 1965 Jun;95:587–596. doi: 10.1042/bj0950587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWEN W. J., KERWIN T. D. The kinetics of myokinase. II. Studies of heat denaturation, the effects of salts and the state of equilibrium. Arch Biochem Biophys. 1956 Oct;64(2):278–284. doi: 10.1016/0003-9861(56)90270-3. [DOI] [PubMed] [Google Scholar]
- CARAFOLI E., ROSSI C. S., LEHNINGER A. L. CATION AND ANION BALANCE DURING ACTIVE ACCUMULATION OF CA++ AND MG++ BY ISOLATED MITOCHONDRIA. J Biol Chem. 1964 Sep;239:3055–3061. [PubMed] [Google Scholar]
- CARAFOLI E., ROSSI C. S., LEHNINGER A. L. UPTAKE OF ADENINE NUCLEOTIDES BY RESPIRING MITOCHONDRIA DURING ACTIVE ACCUMULATION OF CA++ AND PHOSPHATE. J Biol Chem. 1965 May;240:2254–2261. [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- DIPIETRO D. L. SOME ASPECTS OF D-FRUCTOSE METABOLISM IN RAT LIVER. J Biol Chem. 1964 Dec;239:4051–4055. [PubMed] [Google Scholar]
- DRAHOTA Z., CARAFOLI E., ROSSI C. S., GAMBLE R. L., LEHNINGER A. L. THE STEADY STATE MAINTENANCE OF ACCUMULATED CA++ IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2712–2720. [PubMed] [Google Scholar]
- DRESEL F. I. Some observations on the instability of adenine nucleotides in bird-liver preparations. Biochem J. 1953 Jul;54(4):654–659. doi: 10.1042/bj0540654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F., Simer F. The metabolism of normal and tumour tissue: A method for the measurement of respiratory quotient in serum. Biochem J. 1931;25(4):973–984. doi: 10.1042/bj0250973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGLESTON L. V., HEMS R. Separation of adenosine phosphates by paper chromotography and the equilibrium constant of the myokinase system. Biochem J. 1952 Sep;52(1):156–160. doi: 10.1042/bj0520156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBLE J. L., Jr ACCUMULATION OF CITRATE AND MALATE BY MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2668–2672. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J., NEWSHOLME E. A. CITRATE AS AN INTERMEDIARY IN THE INHIBITION OF PHOSPHOFRUCTOKINASE IN RAT HEART MUSCLE BY FATTY ACIDS, KETONE BODIES, PYRUVATE, DIABETES, AND STARVATION. Nature. 1963 Oct 12;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGAARD N., INESI G., BLANKEN R. R. Glucose oxidation and coezymes in cell-free rat heart homogenates. Arch Biochem Biophys. 1960 Sep;90:31–39. doi: 10.1016/0003-9861(60)90607-x. [DOI] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C., STRIEBICH M. J. Cytochemical studies. VII. Localization of endogenous citrate in rat liver fractions. J Biol Chem. 1956 Oct;222(2):969–977. [PubMed] [Google Scholar]
- HOLTEN D. D., NORDLIE R. C. COMPARATIVE STUDIES OF CATALYTIC PROPERTIES OF GUINEA PIG LIVER INTRA- AND EXTRAMITOCHONDRIAL PHOSPHOENOLPYRUVATE CARBOXYKINASES. Biochemistry. 1965 Apr;4:723–731. doi: 10.1021/bi00880a018. [DOI] [PubMed] [Google Scholar]
- KIZER D. E., COX B., LOVIG C. A., FRANCODEESTRUGO S. ADENYLIC ACID DEAMINASE OF RAT LIVER. J Biol Chem. 1963 Sep;238:3048–3052. [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Considerations concerning the pathways of syntheses in living matter; synthesis of glycogen from non-carbohydrate precursors. Bull Johns Hopkins Hosp. 1954 Jul;95(1):19–33. [PubMed] [Google Scholar]
- KREBS H. A. The use of 'CO2 buffers' in manometric measurements of cell metabolism. Biochem J. 1951 Mar;48(3):349–359. doi: 10.1042/bj0480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Dierks C., Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. doi: 10.1042/bj0930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R. Reduced nicotinamide-adenine dinucleotide as a rate-limiting factor in gluconeogenesis. Biochem J. 1964 Dec;93(3):623–627. doi: 10.1042/bj0930623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MANSOUR T. E., MANSOUR J. M. Effects of serotonin (5-hydroxytryptamine) and adenosine 3',5'-phosphate on phosphofructokinase from the liver fluke Fasciola hepatica. J Biol Chem. 1962 Mar;237:629–634. [PubMed] [Google Scholar]
- MANSOUR T. E. STUDIES ON HEART PHOSPHOFRUCTOKINASE. ACTIVE AND INACTIVE FORMS OF THE ENZYME. J Biol Chem. 1965 May;240:2165–2172. [PubMed] [Google Scholar]
- Meldrum N. U., Roughton F. J. Carbonic anhydrase. Its preparation and properties. J Physiol. 1933 Dec 5;80(2):113–142. doi: 10.1113/jphysiol.1933.sp003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON R. E. Oxidation of C14-labeled carbohydrate intermediates in tumor and normal tissue. Cancer Res. 1951 Aug;11(8):571–584. [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- PASSONNEAU J. V., LOWRY O. H. Phosphofructokinase and the Pasteur effect. Biochem Biophys Res Commun. 1962 Feb 20;7:10–15. doi: 10.1016/0006-291x(62)90134-1. [DOI] [PubMed] [Google Scholar]
- POGELL B. M., MCGILVERY R. W. The proteolytic activation of fructose-1, 6-diphosphatase. J Biol Chem. 1952 May;197(1):293–302. [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- RAMAIAH A., HATHAWAY J. A., ATKINSON D. E. ADENYLATE AS A METABOLIC REGULATOR. EFFECT ON YEAST PHOSPHOFRUCTOKINASE KINETICS. J Biol Chem. 1964 Nov;239:3619–3622. [PubMed] [Google Scholar]
- RECKNAGEL R. O., POTTER V. R. Mechanism of the ketogenic effect of ammonium chloride. J Biol Chem. 1951 Jul;191(1):263–275. [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- SALAS M. L., VINUELA E., SALAS M., SOLS A. CITRATE INHIBITION OF PHOSPHOFRUCTOKINASE AND THE PASTEUR EFFECT. Biochem Biophys Res Commun. 1965 Apr 23;19:371–376. doi: 10.1016/0006-291x(65)90471-7. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., BRENNER B. M. 5'-Nucleotidase of rat liver microsomes. J Biol Chem. 1960 Feb;235:471–474. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G. [Further development of manometric methods (carbonate mixtures)]. Z Naturforsch B. 1960 Jun;15B:364–367. [PubMed] [Google Scholar]
- WARNER H. R. Use of analogue computers in the study of control mechanisms in the circulation. Fed Proc. 1962 Jan-Feb;21:87–91. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. Studies on hexokinase. 1. The hexokinase activity of rat-brain extracts. Biochem J. 1951 Aug;49(3):339–347. doi: 10.1042/bj0490339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENNER C. E., DUNN D. F., WEINHOUSE S. A study of glucose oxidation in whole tissue homogenates. J Biol Chem. 1953 Nov;205(1):409–422. [PubMed] [Google Scholar]


