Abstract
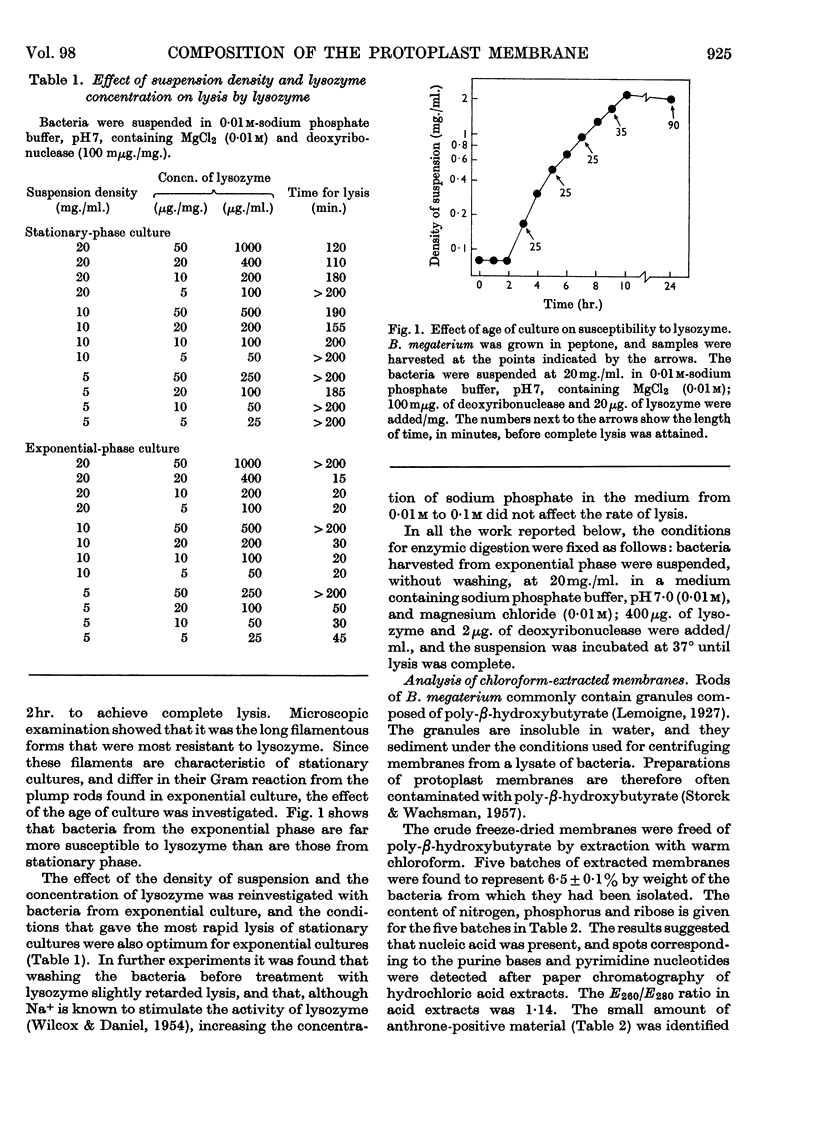
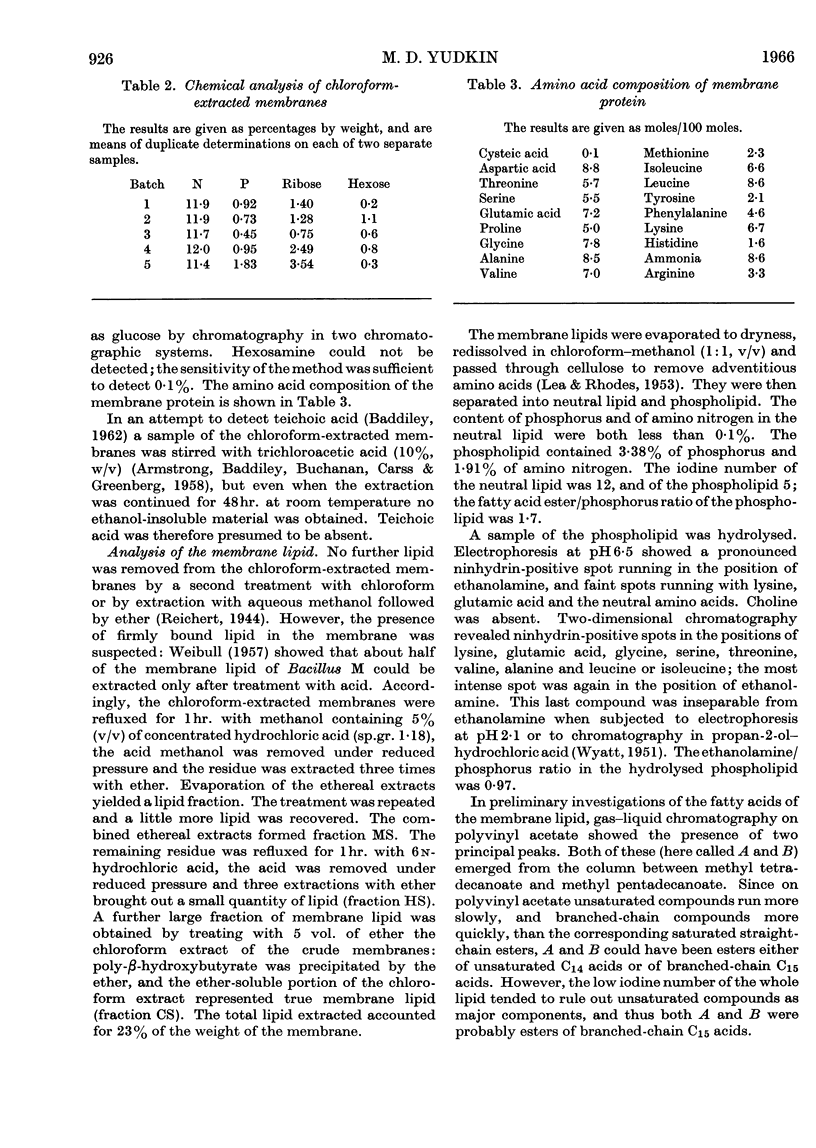
1. Optimum conditions were found for the lysis of Bacillus megaterium KM by lysozyme. The age of culture, density of suspension and concentration of lysozyme affected the rate of lysis. 2. Protoplast membranes were isolated by centrifugation of lysates and were exhaustively washed. 3. Treatment with chloroform removed some lipid from the membranes, but about half of the total membrane lipid could be extracted only after partial acid hydrolysis. 4. The defatted membranes consisted of protein together with variable amounts of RNA; carbohydrate was almost absent. 5. Lipid accounted for 23% of the weight of the membrane, and included both neutral lipid and phospholipid. In both classes, branched-chain C15 acids made up about 80% of the total fatty acid. 6. The phospholipid was a kephalin, and contained small quantities of several amino acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIMER E. H. Studies on L forms and protoplasts of group A streptococci. II. Chemical and immunological properties of the cell membrane. J Exp Med. 1963 Mar 1;117:377–399. doi: 10.1084/jem.117.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABY W. L., HADLEY C., KAMINSKI Z. C. A study of lipides of Penicillium chrysogenum. J Biol Chem. 1957 Aug;227(2):853–861. [PubMed] [Google Scholar]
- GEL'MAN N. S., ZHUKOVA I. G., OPARIN A. I. [Effect of a surface-active substance on the enzymatic system responsible for the oxidation of malic acid in the cytoplasmic membranes of Micrococcus lysodeikticus]. Biokhimiia. 1959 Nov-Dec;24:1074–1078. [PubMed] [Google Scholar]
- GERHARDT P., VENNES J. W. Immunologic comparison of isolated surface membranes of bacillus megaterium. Science. 1956 Sep 21;124(3221):535–536. doi: 10.1126/science.124.3221.535. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., LEYH-BOUILLE M., DIERICKX L. [Structure of the walls of Bacillus megaterium KM. II. Study of the mucopeptide and phosphomucopolysaccharide complexes]. Biochim Biophys Acta. 1962 Sep 24;63:297–307. doi: 10.1016/0006-3002(62)90683-2. [DOI] [PubMed] [Google Scholar]
- GILBY A. R., FEW A. V., McQUILLEN K. The chemical composition of the protoplast membrane of Micrococcus lysodeikticus. Biochim Biophys Acta. 1958 Jul;29(1):21–29. doi: 10.1016/0006-3002(58)90141-0. [DOI] [PubMed] [Google Scholar]
- GODSON G. N., HUNTER G. D., BUTLER J. A. Cellular components of Bacillus megaterium and their role in protein biosynthesis. Biochem J. 1961 Oct;81:59–68. doi: 10.1042/bj0810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W. A model for protein synthesis. Nature. 1962 Mar 3;193:821–823. doi: 10.1038/193821a0. [DOI] [PubMed] [Google Scholar]
- HUNTER G. D., GOODSALL R. A. Lipo-amino acid complexes from Bacillus megaterium and their possible role in protein synthesis. Biochem J. 1961 Mar;78:564–570. doi: 10.1042/bj0780564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T. Gas-liquid partition chromatography: the separation of volatile aliphatic amines and of the homologues of pyridine. Biochem J. 1952 Oct;52(2):242–247. doi: 10.1042/bj0520242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEA C. H., RHODES D. N. Phospholipins. I. Partition chromatography of egg-yolk phospholipins on cellulose. Biochem J. 1953 Jun;54(3):467–469. doi: 10.1042/bj0540467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEA C. H., RHODES D. N. The ninhydrin reaction of unhydrolysed phospholipids. Biochim Biophys Acta. 1955 Jul;17(3):416–423. doi: 10.1016/0006-3002(55)90391-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K., ROBERTS R. B. The utilization of acetate for synthesis in Escherichia coli. J Biol Chem. 1954 Mar;207(1):81–95. [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- SALTON M. R., PAVLIK J. G. Studies of the bacterial cell wall. VI. Wall composition and sensitivity to lysozyme. Biochim Biophys Acta. 1960 Apr 22;39:398–407. doi: 10.1016/0006-3002(60)90191-8. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., BAKAY B., CONOVER M. J., TOENNIES G. Protoplast membrane of Streptococcus faecalis. J Bacteriol. 1963 Jan;85:168–176. doi: 10.1128/jb.85.1.168-176.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORCK R., WACHSMAN J. T. Enzyme localization in Bacillus megaterium. J Bacteriol. 1957 Jun;73(6):784–790. doi: 10.1128/jb.73.6.784-790.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- TUBBS P. K. Effects of inhibitors on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem J. 1962 Jan;82:36–42. doi: 10.1042/bj0820036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C. The nature of the ghosts obtained by lysozyme lysis of Bacillus megaterium. Exp Cell Res. 1956 Feb;10(1):214–221. doi: 10.1016/0014-4827(56)90087-8. [DOI] [PubMed] [Google Scholar]
- WILCOX F. H., Jr, DANIEL L. J. Reduced lysis at high concentrations of lysozyme. Arch Biochem Biophys. 1954 Oct;52(2):305–312. doi: 10.1016/0003-9861(54)90128-9. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUDKIN M. D., DAVIS B. NATURE OF THE RNA ASSOCIATED WITH THE PROTOPLAST MEMBRANE OF BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:193–204. doi: 10.1016/s0022-2836(65)80293-5. [DOI] [PubMed] [Google Scholar]


