Abstract
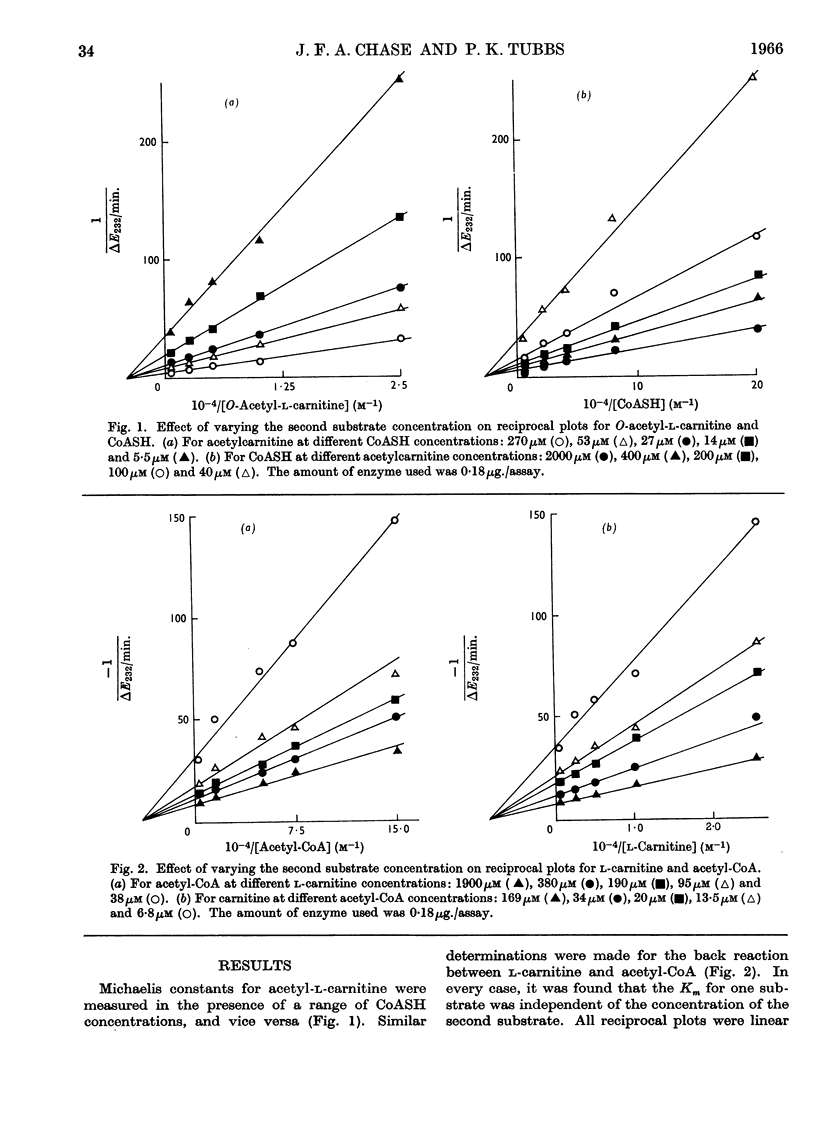
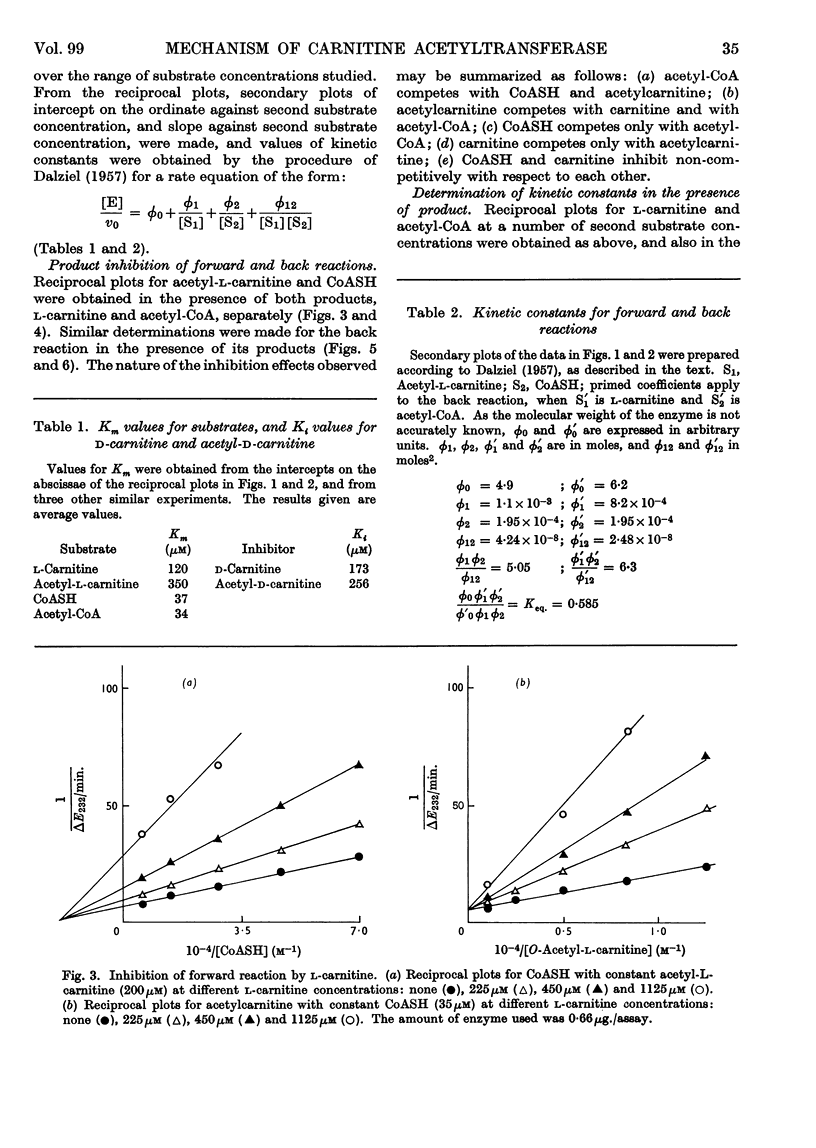
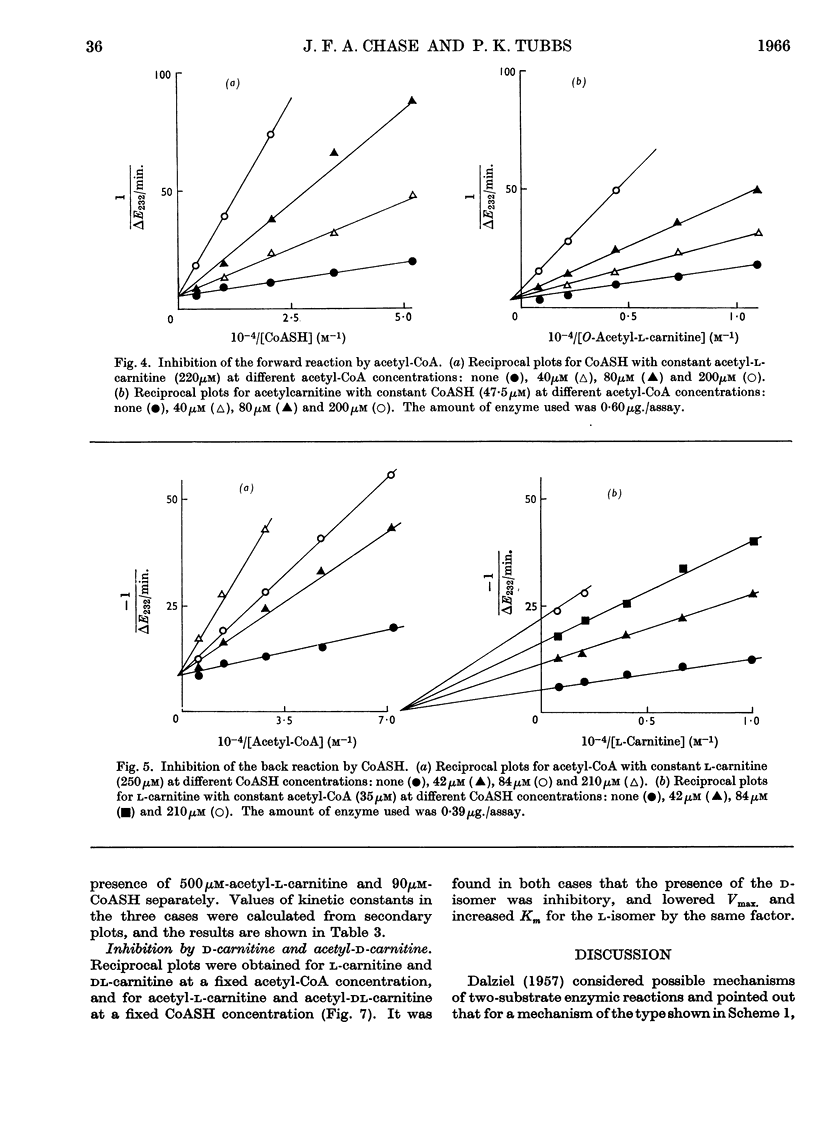
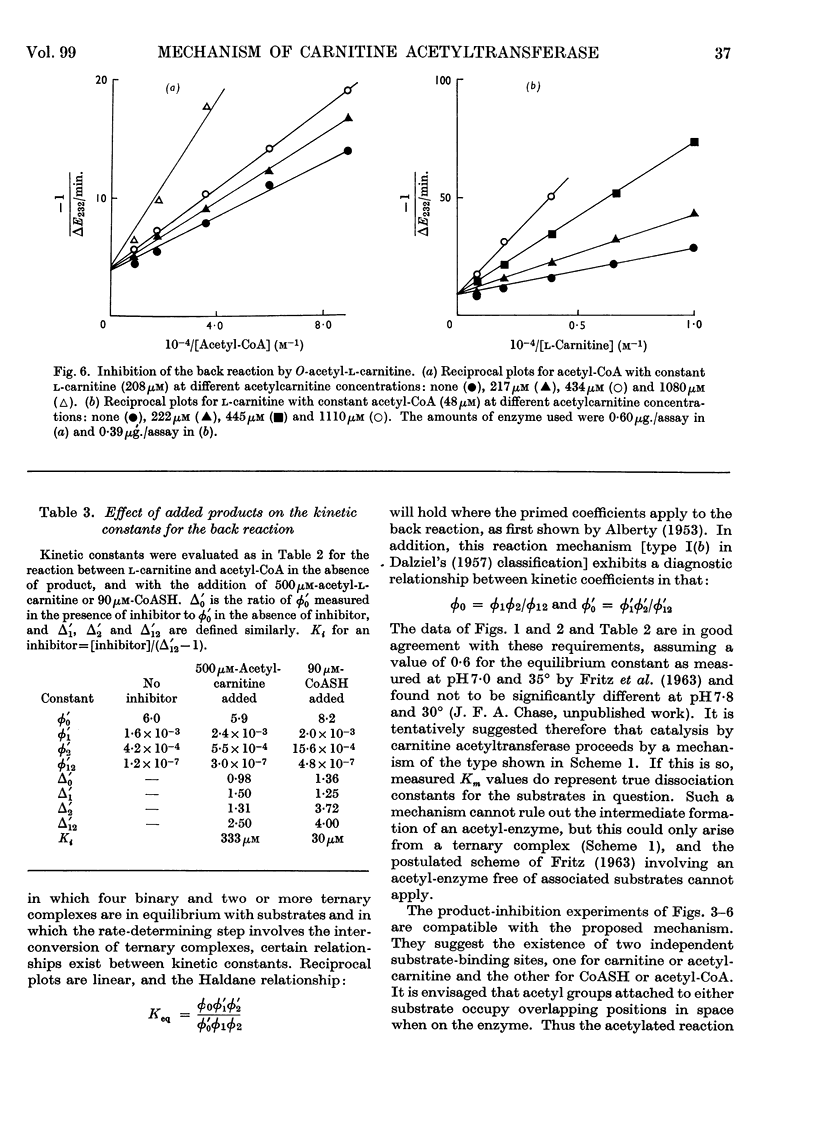
1. Michaelis constants for substrates of carnitine acetyltransferase have been shown to be independent of the concentration of second substrate present. This applies to the forward reaction between acetyl-l-carnitine and CoASH, and to the back reaction between l-carnitine and acetyl-CoA. 2. Product inhibition of both forward and back reactions has been studied. Evidence has been obtained for independent binding sites for l-carnitine and CoASH. Acetyl groups attached to either substrate occupy overlapping positions in space when the substrates are bound to the enzyme. 3. Possible reaction mechanisms involving the ordered addition of substrates have been excluded by determining kinetic constants in the presence and absence of added product. 4. d-Carnitine and acetyl-d-carnitine have been shown to inhibit competitively with respect to l-carnitine and acetyl-l-carnitine. 5. It is concluded that the mechanism of action of carnitine acetyltransferase involves four binary and two or more ternary enzyme complexes in rapid equilibrium with free substrates, the interconversion of the ternary complexes being the rate-limiting step. The possible intermediate formation of an acetyl-enzyme cannot be excluded, but this could only arise from a ternary complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER R. H., Jr, MAHLER H. R. Studies on the mechanism of enzyme-catalyzed oxidation reduction reactions. II. Methods for characterization of the mechanism for two-substrate systems. Biochemistry. 1962 Jan;1:35–40. doi: 10.1021/bi00907a006. [DOI] [PubMed] [Google Scholar]
- CHASE J. F., PEARSON D. J., TUBBS P. K. THE PREPARATION OF CRYSTALLIN CARNITINE ACETYLTRANSFERASE. Biochim Biophys Acta. 1965 Jan;96:162–165. doi: 10.1016/0005-2787(65)90622-2. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FRAENKEL G., FRIEDMAN S. Carnitine. Vitam Horm. 1957;15:73–118. doi: 10.1016/s0083-6729(08)60508-7. [DOI] [PubMed] [Google Scholar]
- FRIEDBERG S. J., BRESSLER R. THE FORMATION AND ISOLATION OF LONG-CHAIN ACYL CARNITINES IN MITOCHONDRIA. Biochim Biophys Acta. 1965 Apr 5;98:335–343. doi: 10.1016/0005-2760(65)90125-6. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K. CARNITINE ACETYLTRANSFERASE. II. INHIBIITON BY CARNITINE ANALOGUES AND BY SULFHYDRYL REAGENTS. J Biol Chem. 1965 May;240:2188–2192. [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- SRERE P. A., KOSICKI G. W. The purification of citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2557–2559. [PubMed] [Google Scholar]
- TUBBS P. K. Effects of inhibitors on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem J. 1962 Jan;82:36–42. doi: 10.1042/bj0820036. [DOI] [PMC free article] [PubMed] [Google Scholar]


