Abstract
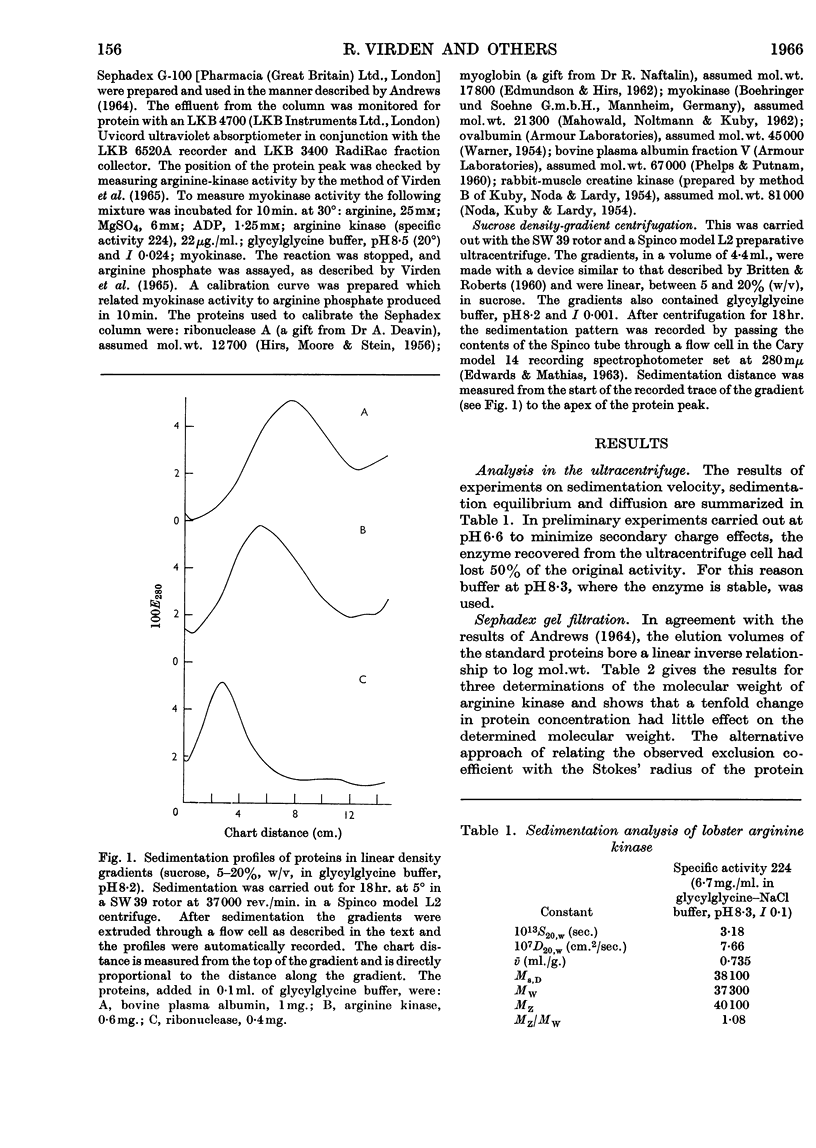
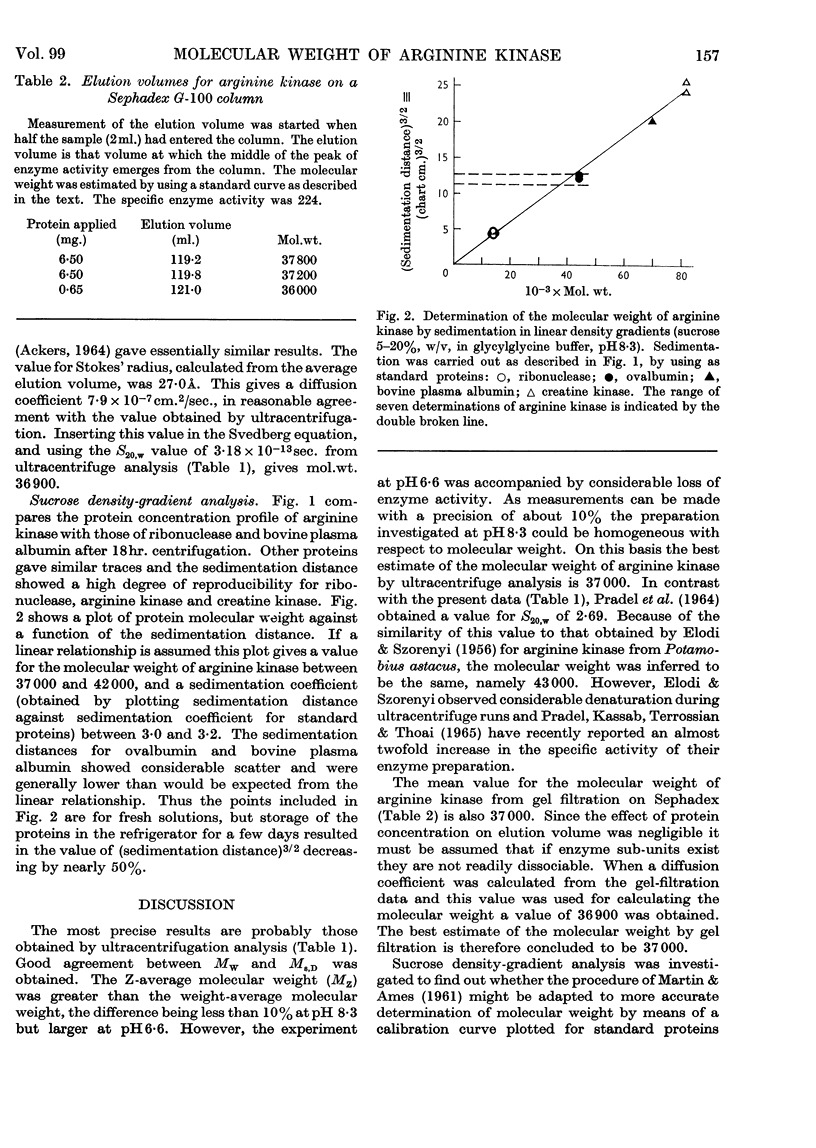
1. The molecular weight of arginine kinase from lobster muscle has been determined by three procedures: ultracentrifuge analysis, gel filtration and density-gradient centrifugation. 2. The three methods give similar results and the best estimate of the molecular weight is 37000. 3. The enzyme does not readily show association–dissociation phenomena. 4. The usefulness of density-gradient centrifugation for determinations of molecular weight is briefly discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- EDWARDS J. G., MATHIAS A. P. APPARATUS FOR THE AUTOMATIC LOCATION OF ABSORBING COMPONENTS SEPARATED BY DENSITY GRADIENT CENTRIFUGATION. Nature. 1963 Aug 10;199:603–604. doi: 10.1038/199603a0. [DOI] [PubMed] [Google Scholar]
- ELODI P., SZORENYI E. Properties of crystalline arginine-phosphoferase isolated from Crustacean muscle. Acta Physiol Acad Sci Hung. 1956;9(4):367–379. [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- MAHOWALD T. A., NOLTMANN E. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. I. Amino acid composition of adenosine triphosphate-adenosine 5'-phosphate transphosphorylase (myokinase). J Biol Chem. 1962 Apr;237:1138–1145. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. II. Homogeneity and physicochemical properties. J Biol Chem. 1954 Jul;209(1):203–210. [PubMed] [Google Scholar]
- PRADEL L. A., KASSAB R., REGNOUF F., NGUYENVAN THOAI SITES ACTIFS DE L'ATP: ARGININE PHOSPHOTRANSF'ERASE. Biochim Biophys Acta. 1964 Aug 26;89:255–265. [PubMed] [Google Scholar]
- VIRDEN R., WATTS D. C., BALDWIN E. ADENOSINE 5'-TRIPHOSPHATE-ARGININE PHOSPHOTRANSFERASE FROM LOBSTER MUSCLE: PURIFICATION AND PROPERTIES. Biochem J. 1965 Mar;94:536–544. doi: 10.1042/bj0940536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRDEN R., WATTS D. C. THE DISTRIBUTION OF GUANIDINE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES AND ADENOSINE TRIPHOSPHATASE IN ANIMALS FROM SEVERAL PHYLA. Comp Biochem Physiol. 1964 Oct;13:161–177. doi: 10.1016/0010-406x(64)90202-6. [DOI] [PubMed] [Google Scholar]
- Virden R., Watts D. C. Adenosine 5'-triphosphate-arginine phosphotransferase from lobster muscle. Biochem J. 1966 Apr;99(1):159–161. doi: 10.1042/bj0990159. [DOI] [PMC free article] [PubMed] [Google Scholar]


