Abstract
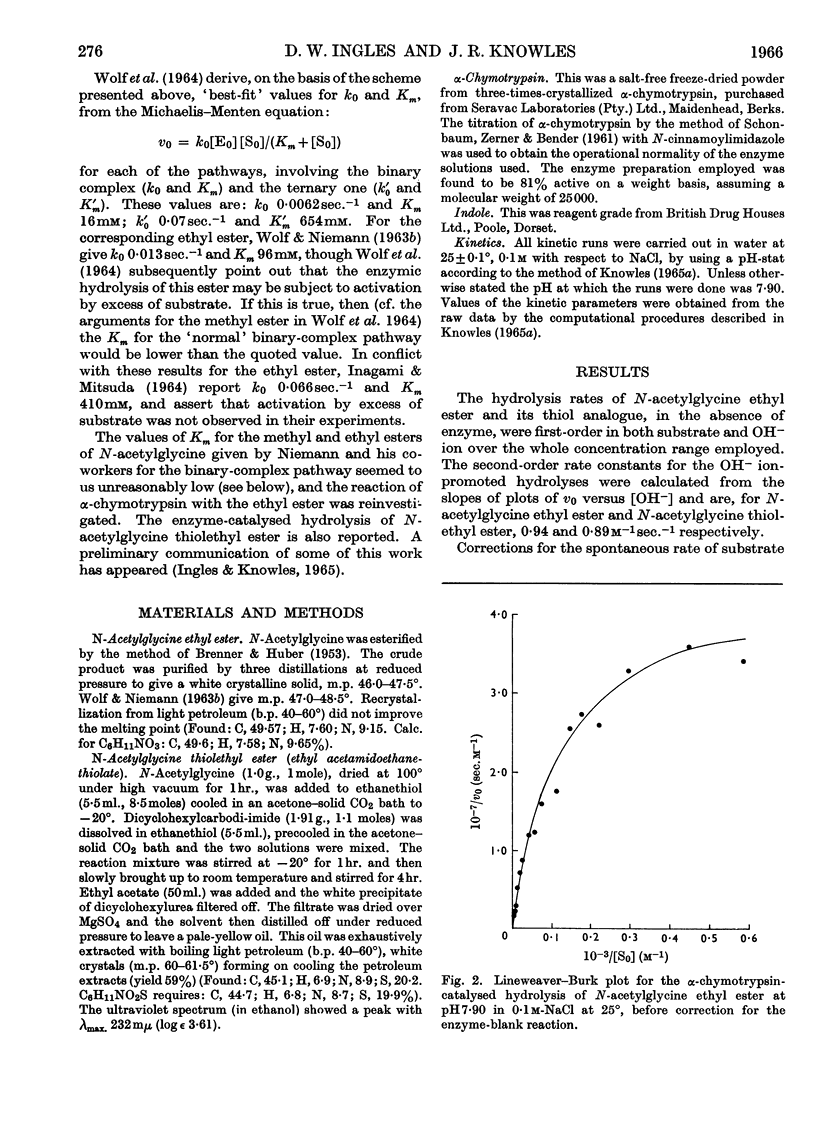
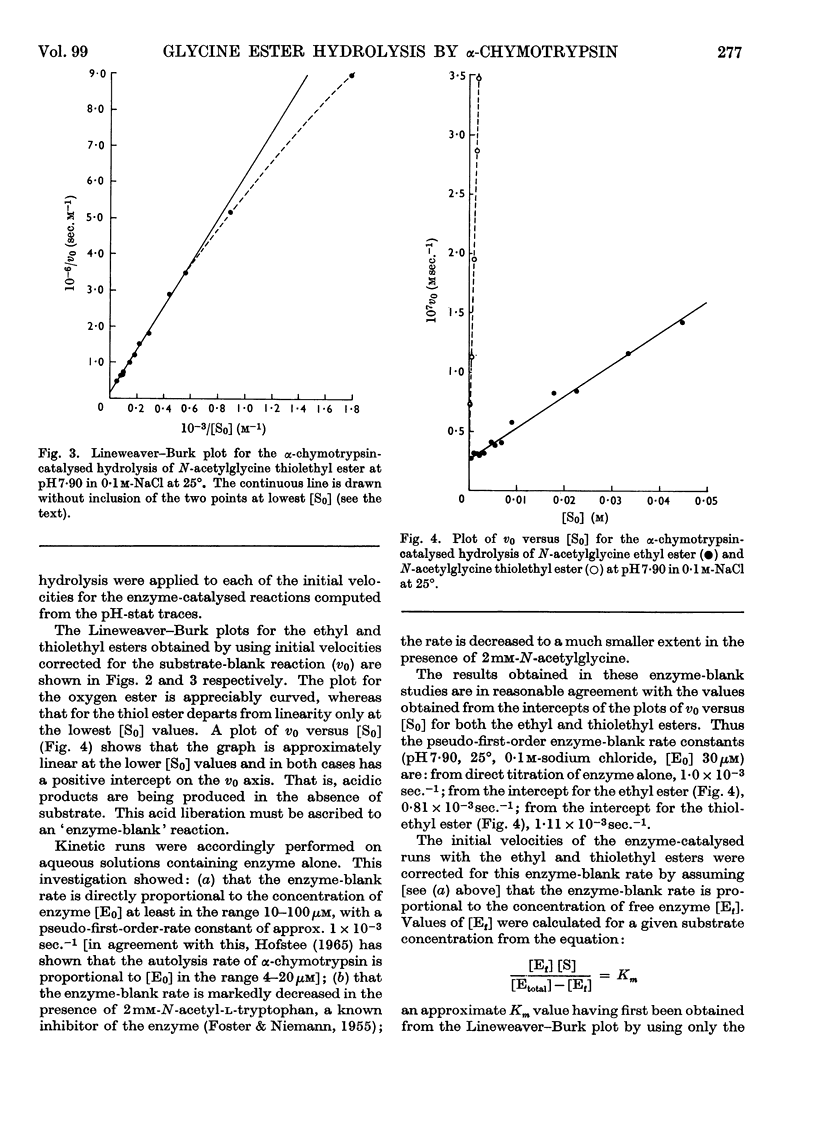
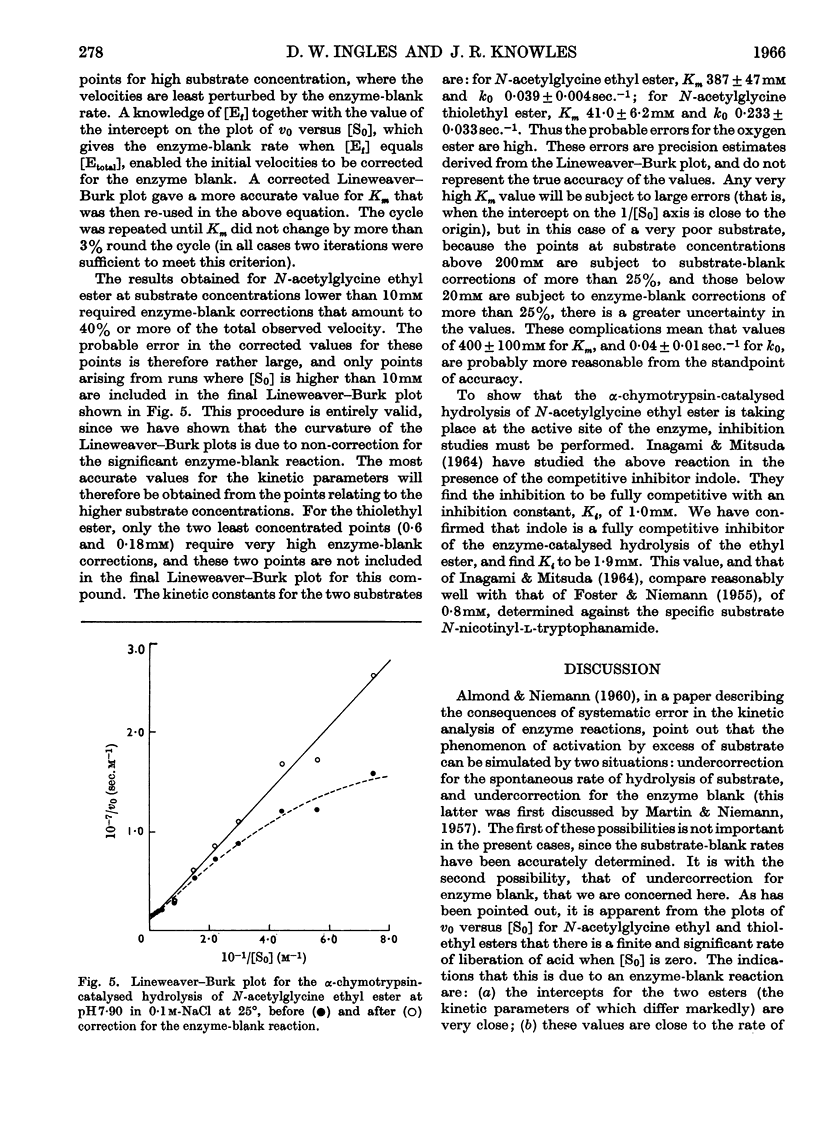
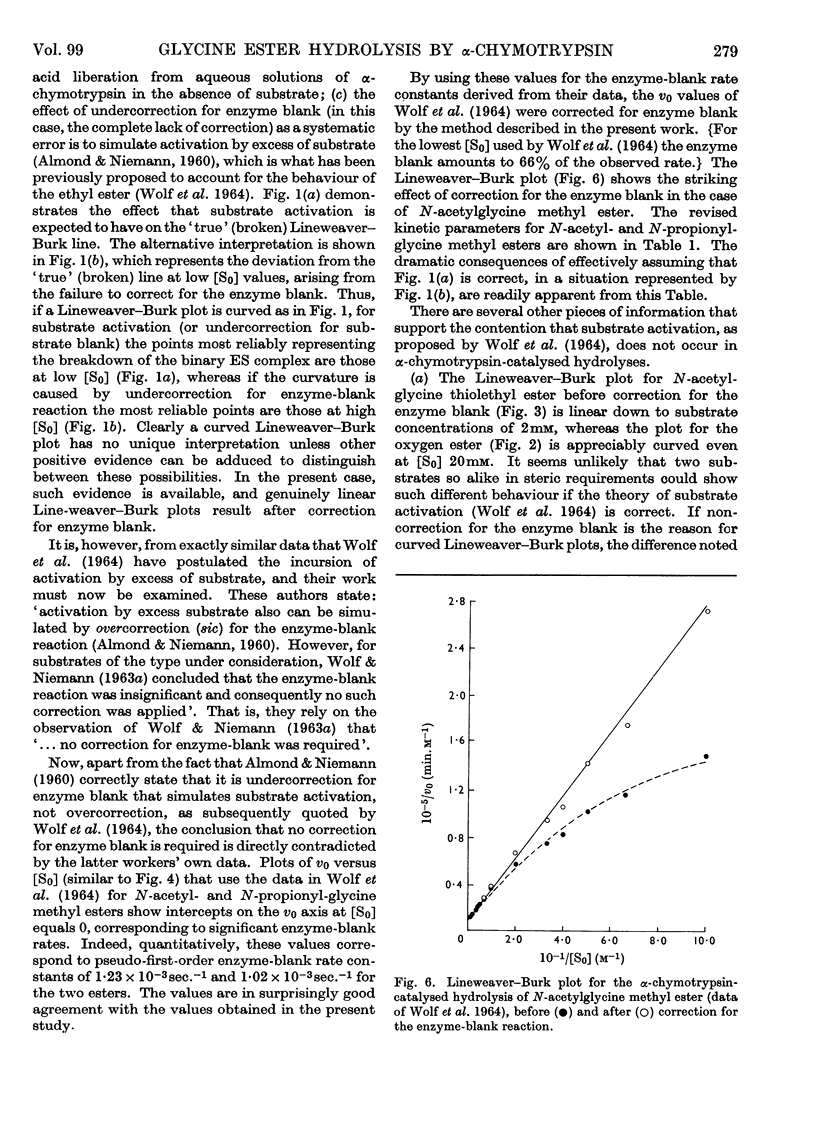
1. The α-chymotrypsin-catalysed hydrolysis of N-acetylglycine ethyl and thiolethyl esters was investigated at pH7·90 and 25° over a wide range of substrate concentrations. 2. The Lineweaver–Burk plots for these substrates are markedly curved, and it is shown that the curvature is due solely to the `enzyme-blank' reaction. The rate of this reaction is proportional to free enzyme concentration in the range 10–100μm, with a pseudo-first-order rate constant of approx. 1×10−3sec.−1. Correction for this reaction by the procedure described leads to linear plots. It is shown that the significance of the enzyme-blank reaction depends on the value of k0/Km for the substrate under investigation. 3. Interpretation of the curvature in the Lineweaver–Burk plots by previous workers in terms of activation by excess of substrate is shown to be erroneous. 4. Values of Km 387mm and k0 0·039sec.−1, and Km 41mm and k0 0·23sec.−1, were obtained for the ethyl and thiolethyl esters of N-acetylglycine respectively. The literature values for the methyl esters of N-acetyl- and N-propionyl-glycine have been corrected by the procedure described. The new values agree much better with current theories of α-chymotrypsin mechanism and specificity. 5. The kinetic parameters for the ethyl and thiolethyl esters indicate the absence of an electrophilic component in the catalytic mechanism of α-chymotrypsin, and the importance of the ester function in substrate binding.
Full text
PDF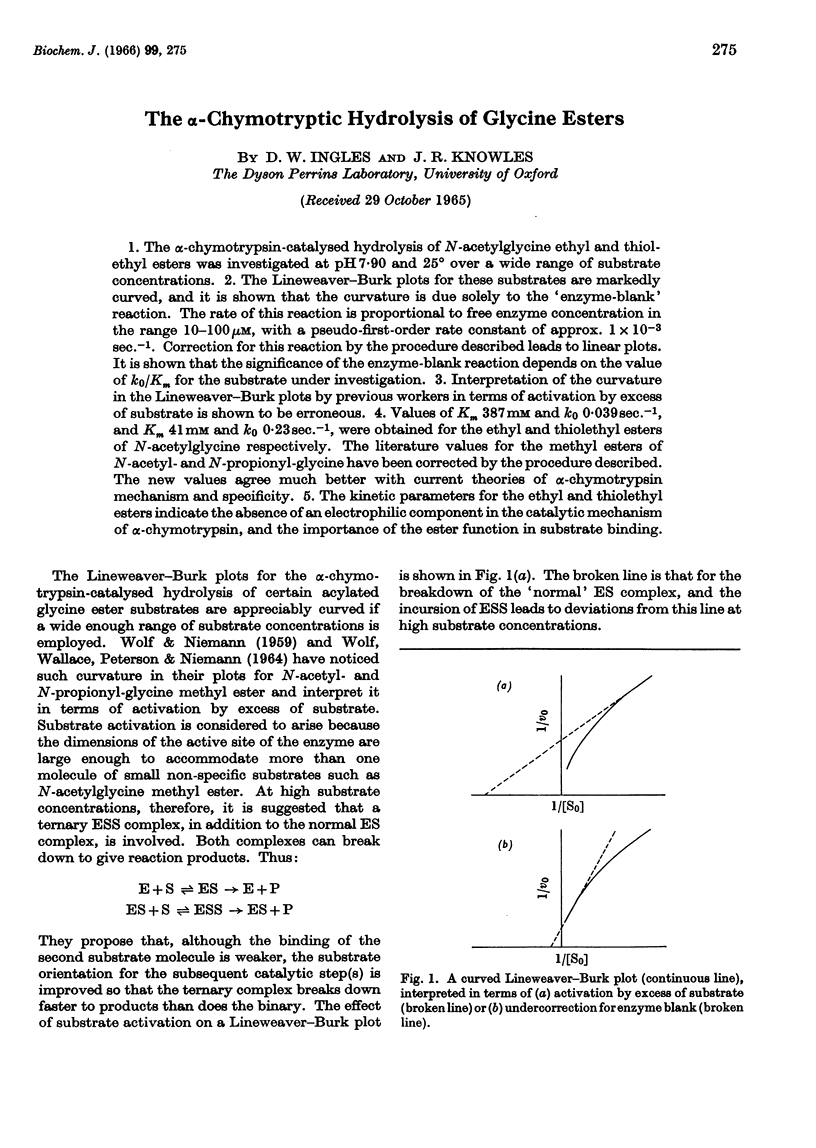
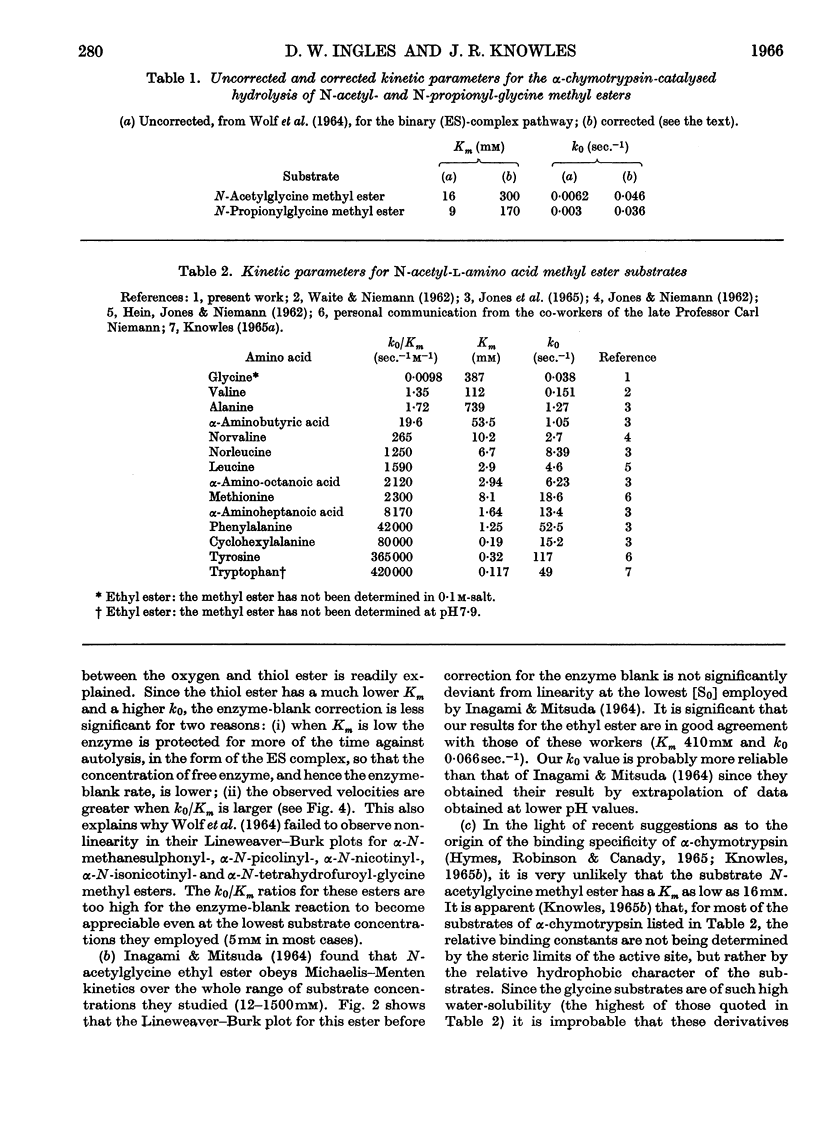


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMOND H. R., Jr, NIEMANN C. The consequences of systematic error in enzyme kinetics. Biochim Biophys Acta. 1960 Oct 21;44:143–150. doi: 10.1016/0006-3002(60)91532-8. [DOI] [PubMed] [Google Scholar]
- HEIN G. E., JONES J. B., NIEMANN C. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of acetyl-L-leucine methyl ester. Biochim Biophys Acta. 1962 Dec 4;65:353–355. doi: 10.1016/0006-3002(62)91056-9. [DOI] [PubMed] [Google Scholar]
- HEIN G., NIEMANN C. An interpretation of the kinetic behavior of model substrates of alpha-chymotrypsin. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1341–1355. doi: 10.1073/pnas.47.9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYMES A. J., ROBINSON D. A., CANADY W. J. THERMODYNAMICS OF THE SOLUTION PROCESS. II. THE USE OF AN EXTRACTION MODEL FOR ENZYME-INHIBITOR COMPLEX FORMATION. J Biol Chem. 1965 Jan;240:134–138. [PubMed] [Google Scholar]
- INAGAMI T., MITSUDA H. THE MECHANISM OF THE SPECIFICITY OF TRYPSIN CATALYSIS. II. COMPARISON OF TRYPSIN AND ALPHA-CHYMOTRYPSIN IN THE NONSPECIFIC CATALYSES OF TEH HYDROLYSIS OF ACETYLGLYCINE ETHYL ESTER. J Biol Chem. 1964 May;239:1388–1394. [PubMed] [Google Scholar]
- JONES J. B., KUNITAKE T., NIEMANN C., HEIN G. E. THE PRIMARY SPECIFICITY OF ALPHA-CHYMOTRYPSIN. ACYLATED AMINO ACID ESTERS WITH NORMAL ALKYL SIDE CHAINS. J Am Chem Soc. 1965 Apr 20;87:1777–1781. doi: 10.1021/ja01086a029. [DOI] [PubMed] [Google Scholar]
- KNOWLES J. R. THE ROLE OF METHIONINE IN ALPHA-CHYMOTRYPSIN-CATALYSED REACTIONS. Biochem J. 1965 Apr;95:180–190. doi: 10.1042/bj0950180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard G. E., Jencks W. P. The reaction of carbanions with N,S-diacetylcysteamine. A model for enzymatic carbon--carbon condensation. J Am Chem Soc. 1965 Sep 5;87(17):3863–3874. doi: 10.1021/ja01095a013. [DOI] [PubMed] [Google Scholar]
- MARTIN R. B., NIEMANN C. The nature of the enzyme blank observed with crystalline alpha-chymotrypsin. Biochim Biophys Acta. 1957 Dec;26(3):634–635. doi: 10.1016/0006-3002(57)90110-5. [DOI] [PubMed] [Google Scholar]
- SCHONBAUM G. R., ZERNER B., BENDER M. L. The spectrophotometric determination of the operational normality of an alpha-chymotrypsin solution. J Biol Chem. 1961 Nov;236:2930–2935. [PubMed] [Google Scholar]
- WOLF J. P., 3rd, NIEMANN C. THE ALPHA-CHYMOTRYPSIN-CATALYZED HYDROLYSIS OF A SERIES OF ANALOGS OF ACETYLGLYCINE METHYL ESTER. Biochemistry. 1963 May-Jun;2:493–497. doi: 10.1021/bi00903a017. [DOI] [PubMed] [Google Scholar]
- WOLF J. P., 3rd, WALLACE R. A., PETERSON R. L., NIEMANN C. THE ALPHA-CHYMOTRYPSIN-CATALYZED HYDROLYSIS OF A SERIES OF ACYLATED GLYCINE METHYL ESTERS. II. BEHAVIOR AT LOW AND HIGH SUBSTRATE CONCENTRATIONS. Biochemistry. 1964 Jul;3:940–944. doi: 10.1021/bi00895a016. [DOI] [PubMed] [Google Scholar]


