Abstract
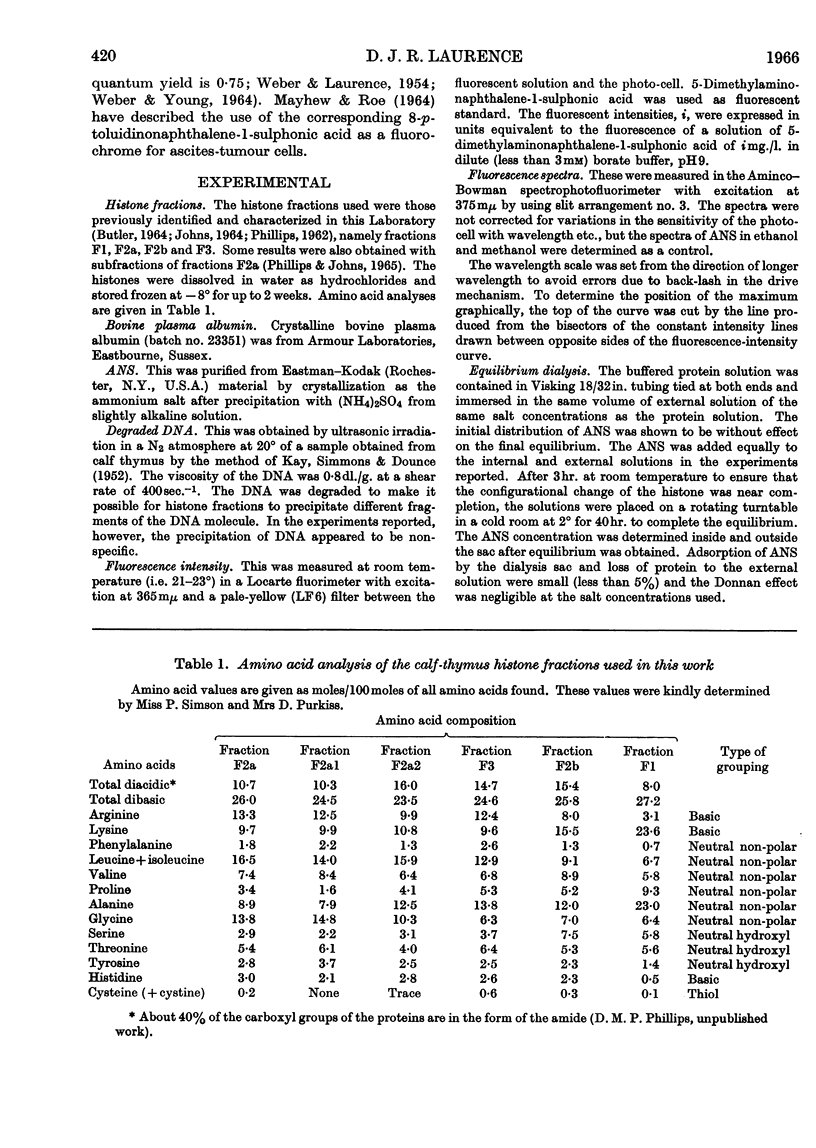
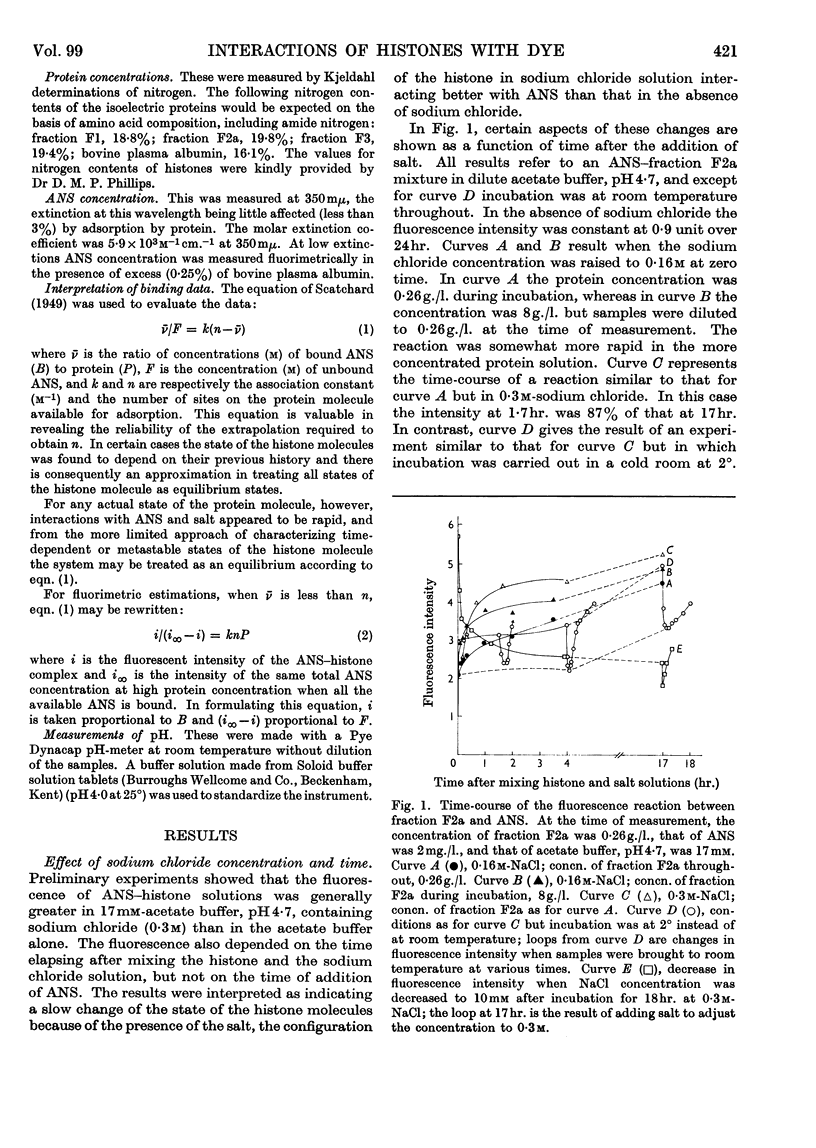
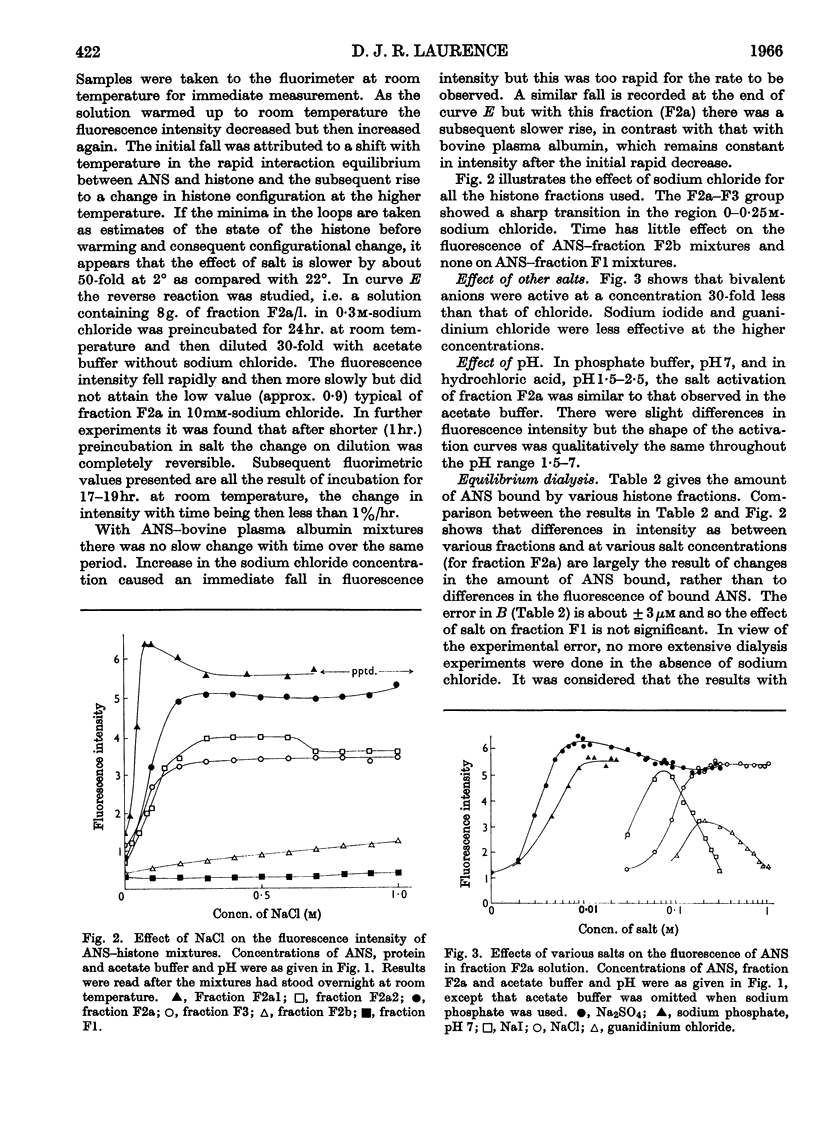
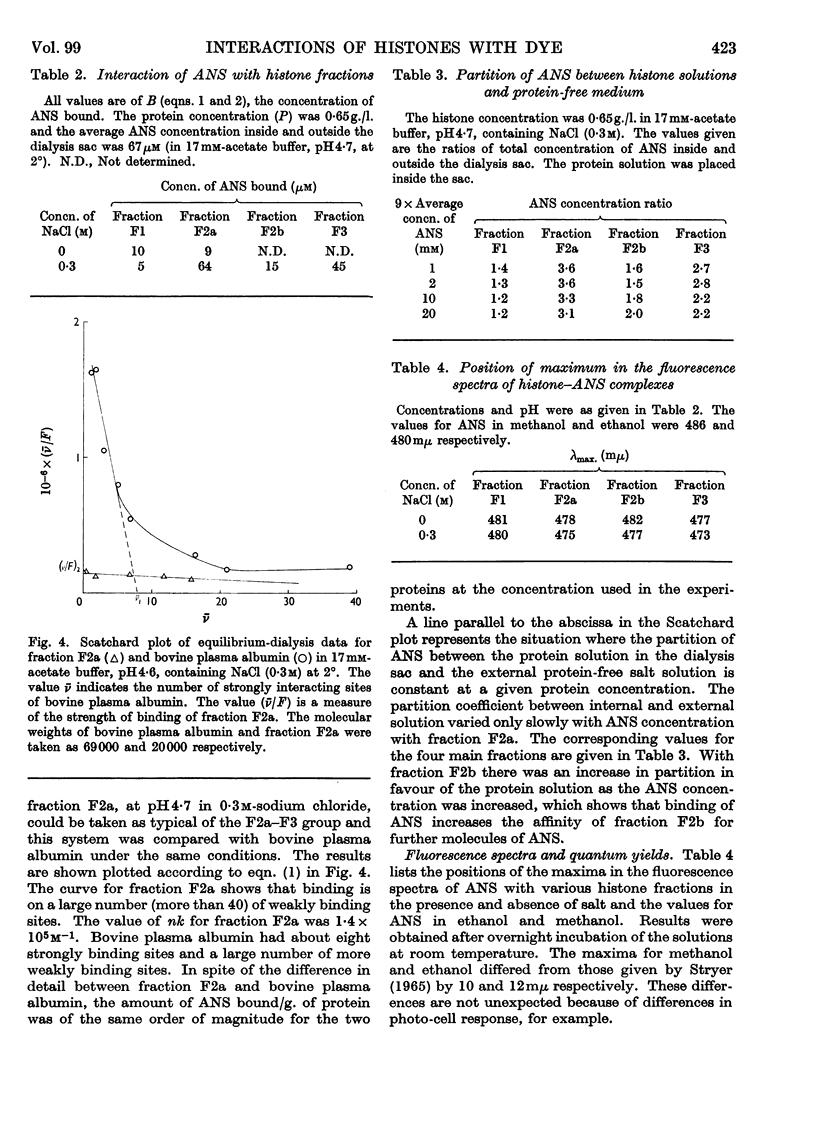
1. The interactions of histone fractions with 8-anilinonaphthalene-1-sulphonic acid were investigated by fluorimetry and spectrofluorimetry and the results were interpreted with the aid of equilibrium-dialysis techniques. 2. Characteristic differences were found between the various histone fractions, and with fractions F3 and F2a the binding was found to be salt-dependent. 3. Evidence was obtained indicating a slow change of the physical state of fractions F3 and F2a in the presence of salt, and the binding by these two fractions in the presence of salt was greater by an order of magnitude than by fractions F1 and F2b. 4. Conditions favouring binding were also those favouring histone aggregation; SO42− ions activated binding at a lower concentration than Cl− ions; urea, guanidinium ions and high concentrations of I− ions were inhibitory to binding. 5. After histones had been kept in the presence of salt for a long time the reversal of interaction on decreasing the salt concentration was incomplete. 6. The inhibition of binding by fraction F2a in the presence of urea or fraction F2b depended on the time sequence of addition of the reagents. 7. Artificial nucleoproteins made by precipitating DNA with the histone fractions in neutral 0·14m-sodium chloride showed the same order of interaction as was found for the fractions in solution. 8. Comparison of the binding by fraction F2a with that by bovine plasma albumin showed that in both cases there were a large number of weakly binding sites but that fraction F2a lacked the small number of strongly binding sites found in albumin. No slow change of binding in the presence of salt was found for albumin. 9. Binding by fraction F2b increased the affinity of the protein for further molecules of the adsorbate. 10. The results are discussed in relation to the close relationship between binding and aggregation and the possible role of non-polar interactions as determined by the balance between polar and non-polar amino acids in the histone fractions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfert M., Geschwind I. I. A Selective Staining Method for the Basic Proteins of Cell Nuclei. Proc Natl Acad Sci U S A. 1953 Oct;39(10):991–999. doi: 10.1073/pnas.39.10.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENCE D. J. R. A study of the adsorption of dyes on bovine serum albumin by the method of polarization of fluorescence. Biochem J. 1952 May;51(2):168–180. doi: 10.1042/bj0510168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB J. ETUDE DE LA M'ETACHROMASIE DE L''EOSINE EN PR'ESENCE D'HISTONES. C R Hebd Seances Acad Sci. 1964 May 20;258:5087–5090. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAURITZEN C. M., STEDMAN E. Cell specificity of beta-histones in the domestic fowl. Proc R Soc Lond B Biol Sci. 1959 Apr 21;150(940):299–311. doi: 10.1098/rspb.1959.0023. [DOI] [PubMed] [Google Scholar]
- MAYHEW E., ROE E. M. CHANGES IN THE PERMEABILITY OF LANDSCHUETZ ASCITES TUMOUR CELLS TO VITAL STAINS AFTER TREATMENT WITH TUMOUR-INHIBITORY OR MODIFIED SAMPLES OF GUM TRAGACANTH OR WITH GUM KARAYA. Br J Cancer. 1964 Sep;13:537–542. doi: 10.1038/bjc.1964.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY K. THE BASIC PROTEINS OF CELL NUCLEI. Annu Rev Biochem. 1965;34:209–246. doi: 10.1146/annurev.bi.34.070165.001233. [DOI] [PubMed] [Google Scholar]
- PHILLIPS D. M., JOHNS E. W. A FRACTIONATION OF THE HISTONES OF GROUP F2A FROM CALF THYMUS. Biochem J. 1965 Jan;94:127–130. doi: 10.1042/bj0940127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. R., Noland B. J. A fluorescence assay suitable for histone solutions. Anal Biochem. 1965 Jun;11(3):443–448. doi: 10.1016/0003-2697(65)90063-1. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- UI N. On the molecular weight of calf thymus histone. Biochim Biophys Acta. 1956 Oct;22(1):205–206. doi: 10.1016/0006-3002(56)90249-9. [DOI] [PubMed] [Google Scholar]
- WEBER G., LAURENCE D. J. Fluorescent indicators of adsorption in aqueous solution and on the solid phase. Biochem J. 1954 Jan 16;56(325TH):xxxi–xxxi. [PubMed] [Google Scholar]
- WEBER G., YOUNG L. B. FRAGMENTATION OF BOVINE SERUM ALBUMIN BY PEPSIN. I. THE ORIGIN OF THE ACID EXPANSION OF THE ALBUMIN MOLECULE. J Biol Chem. 1964 May;239:1415–1423. [PubMed] [Google Scholar]


