Abstract
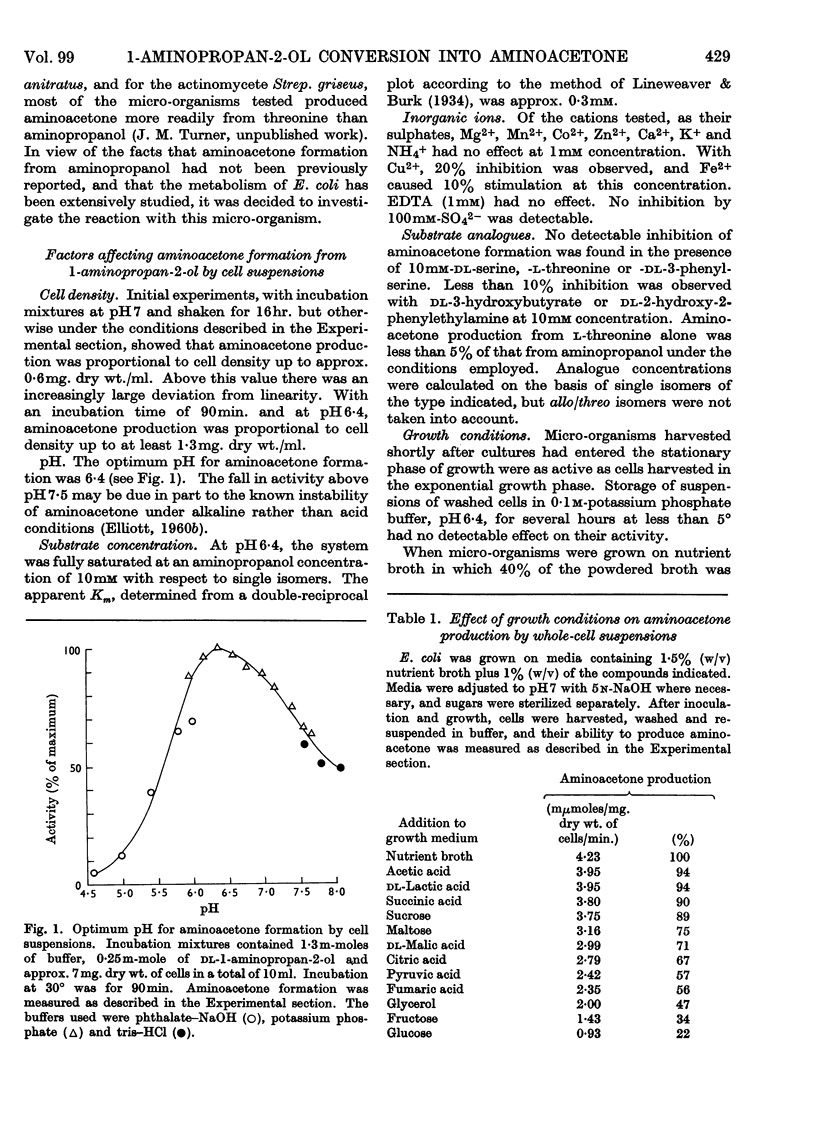
1. Washed-cell suspensions of Escherichia coli, incubated at the optimum pH of 6·4 and with a saturating substrate concentration of approx. 10mm, convert dl-1-aminopropan-2-ol into aminoacetone at a rate of approx. 4·0mμmoles/mg. dry wt. of cells/min. at 30°. 2. Mg2+, Mn2+, Co2+, Zn2+, Ca2+, K+ and NH4+, as sulphates, and EDTA have no effect on this rate, although Cu2+ inhibits and Fe2+ activates to some extent. 3. Conditions of growth markedly affect the rate of aminoacetone production by cell suspensions. 4. Dialysed cell-free extracts of E. coli exhibit 1-aminopropan-2-ol-dehydrogenase activity, the enzyme having optimum activity at pH7·0, a requirement for NAD+ and K+, and a Km for the amino alcohol substrate of 0·8mm, calculated for a single enantiomorph. 5. Under optimum conditions 1-aminopropan-2-ol dehydrogenase forms aminoacetone at rate of approx. 3·0mμmoles/mg. of protein/min. at 37°. The enzyme is only slightly inhibited by dl-3-hydroxybutyrate and dl-2-hydroxy-2-phenylethyl-amine. 6. l-Threonine-dehydrogenase activity is exhibited by both whole cells and cell-free extracts. Whole cells produce aminoacetone from l-threonine more slowly than they do from dl-1-aminopropan-2-ol, whereas the situation is reversed in cell-free extracts. Both kinetic evidence, and the fact that synthesis of 1-aminopropan-2-ol dehydrogenase, but not of threonine dehydrogenase, is repressed by compounds such as glucose and pyruvate, provide evidence that the amino alcohol is oxidized by a specific enyme. 7. The metabolic role of 1-aminopropan-2-ol dehydrogenase is discussed.
Full text
PDF

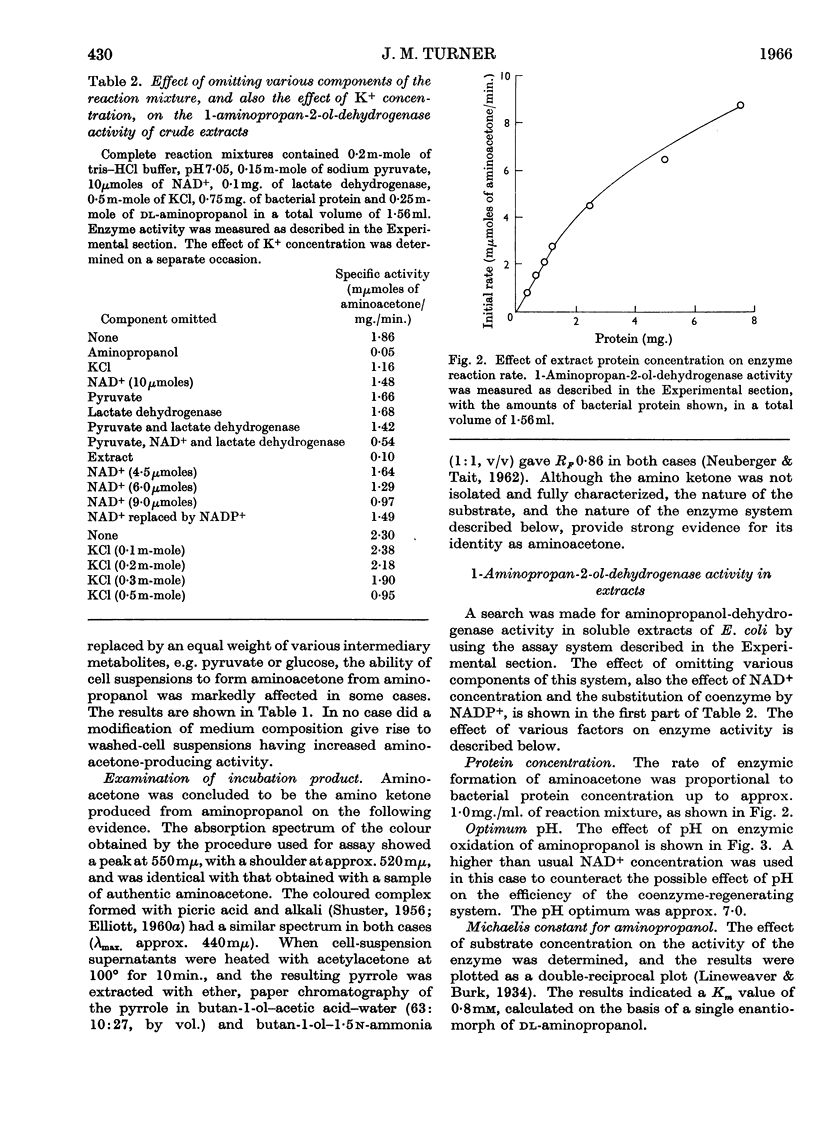
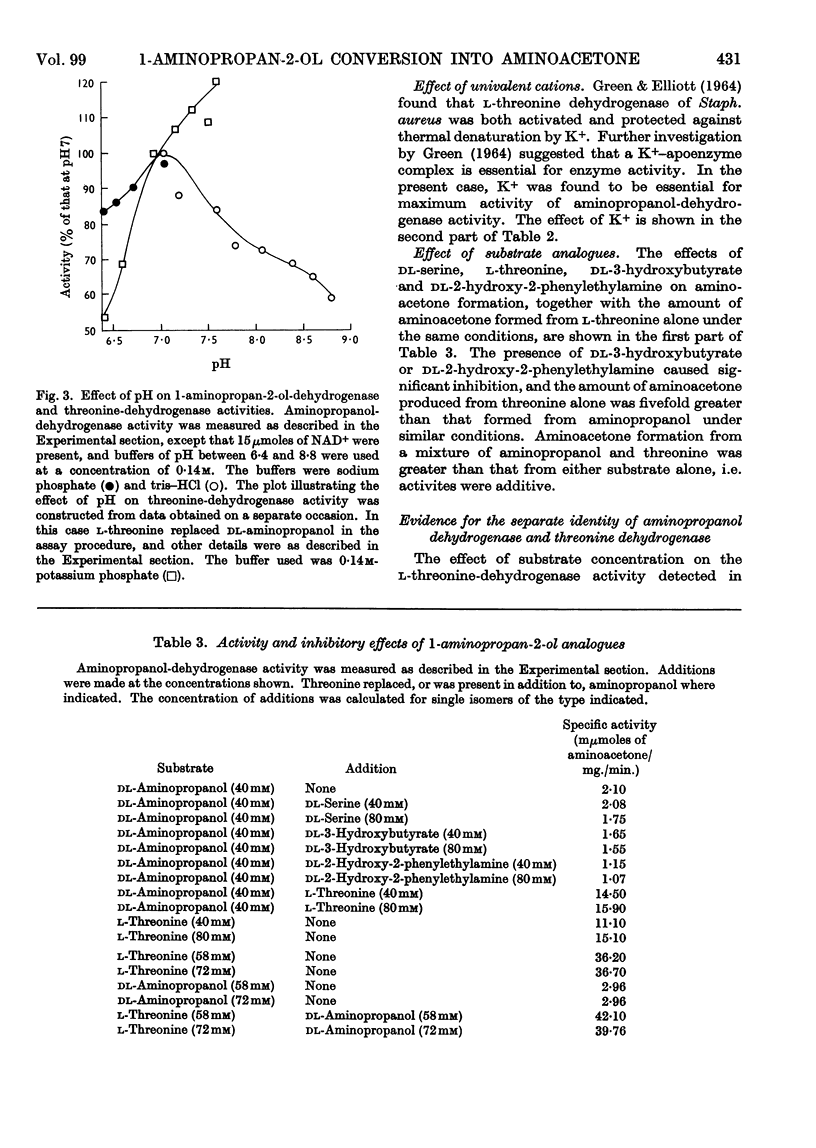

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLIOTT W. H. Amino-acetone; its isolation and role in metabolism. Nature. 1959 Apr 11;183(4667):1051–1052. doi: 10.1038/1831051a0. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. H. Aminoacetone formation by Staphylococcus aureus. Biochem J. 1960 Mar;74:478–485. doi: 10.1042/bj0740478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. The estimation of aminoacetone and 5-aminolaevulic acid. Biochem J. 1960 Jan;74:90–94. doi: 10.1042/bj0740090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEB S. F., MANDEL M. Utilization of 1-amino-2-propanol by a soil bacterium. Can J Microbiol. 1959 Aug;5:363–368. doi: 10.1139/m59-045. [DOI] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. L. The activation of L-threonine dehydrogenase by potassium ions. Biochem J. 1964 Sep;92(3):550–555. doi: 10.1042/bj0920550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTSHORNE D., GREENBERG D. M. STUDIES ON LIVER THREONINE DEHYDROGENASE. Arch Biochem Biophys. 1964 Apr;105:173–178. doi: 10.1016/0003-9861(64)90250-4. [DOI] [PubMed] [Google Scholar]
- KRASNA A. I., ROSENBLUM C., SPRINSON D. B. The conversion of L-threonine to the Dg-1-amino-2-propanol of vitamin B12. J Biol Chem. 1957 Apr;225(2):745–750. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- NEUBERGER A. Aspects of the metabolism of glycine and of porphyrins. Biochem J. 1961 Jan;78:1–10. doi: 10.1042/bj0780001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. Production of aminoacetone by Rhodopseudomonas spheroides. Biochem J. 1962 Aug;84:317–328. doi: 10.1042/bj0840317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. The enzymic conversion of threonine to aminoacetone. Biochim Biophys Acta. 1960 Jun 17;41:164–165. doi: 10.1016/0006-3002(60)90388-7. [DOI] [PubMed] [Google Scholar]
- SHUSTER L. The determination of delta -aminolaevulic acid. Biochem J. 1956 Sep;64(1):101–106. doi: 10.1042/bj0640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]


