Abstract
Background
Delirium is common and often goes undetected in older patients admitted to medical services. It is associated with poor outcomes. We conducted a randomized clinical trial to determine whether systematic detection and multidisciplinary care of delirium in older patients admitted to a general medical service could reduce time to improvement in cognitive status.
Methods
Consecutive patients aged 65 or more who were newly admitted to 5 general medical units between Mar. 15, 1996, and Jan. 31, 1999, were screened with the Confusion Assessment Method within 24 hours after admission to detect prevalent delirium and rescreened within a week to detect incident cases. Patients with delirium were randomly allocated to receive the intervention or usual care. Subjects in the intervention group were seen by a geriatric specialist consultant and followed in hospital for up to 8 weeks by an intervention nurse who liaised with the consultant, attending physicians, family and the primary care nurses. Subjects in the usual care group received standard hospital services but could consult geriatric specialists as needed. A research assistant, blinded as to treatment allocation, administered within 24 hours after enrolment the Mini-Mental Status Exam (MMSE), Delirium Index (measuring the severity of the delirium) and Barthel Index (measuring independence of personal care). Improvement was defined as an increase in the MMSE score of 2 or more points, with no decrease below baseline plus 2 points, or no decrease below a baseline MMSE score of 27. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly was completed to identify patients with possible dementia. Subjects were assessed 3 times during the first week and weekly thereafter for up to 8 weeks in hospital or until discharge. Data on clinical severity of illness, length of stay and living arrangements after discharge were also collected. The primary outcome measure was time to improvement in MMSE score.
Results
Of the 1925 patients who met the inclusion criteria and were screened, 227 had prevalent or incident delirium and consented to participate (113 in intervention group and 114 in usual care group). There were no clinically significant differences between the intervention and usual care groups except for sex (female 58.4% v. 50.0%) and marital status (married 34.8% v. 41.2%). Overall, 48% of the patients in the intervention group and 45% of those in the usual care group met the predetermined criteria for improvement. The Cox proportional hazards ratio (HR) for a shorter time to improvement with the intervention versus usual care, adjusted for age, sex and marital status, was 1.10 (95% confidence interval [CI] 0.74–1.63). There were no significant differences within 8 weeks after enrolment between the 2 groups in time to and rate of improvement of the Delirium Index, the Barthel Index, length of stay, rate of discharge to the community, living arrangements after discharge or survival. Outcomes between the 2 groups did not differ statistically significantly for patients without dementia (HR 1.54, 95% CI 0.80–2.97), for those who had less comorbidity (HR 1.36, 95% CI 0.75–2.46) or for those with prevalent delirium (HR 1.15, 95% CI 0.48–2.79).
Interpretation
Systematic detection and multidisciplinary care of delirium does not appear to be more beneficial than usual care for older patients admitted to medical services.
Delirium is a mental disorder characterized by acute onset, an altered level of consciousness, a fluctuating course and disturbances in orientation, memory, attention, thinking, perception and behaviour.1,2 It occurs in 11%–26% of older patients admitted to general medical units3,4,5,6,7,8,9,10 and appears to be associated with significant increases in length of hospital stay, rates of admission to a long-term care facility functional disability and rates of death.3,11,12,13,14,15,16,17 Despite the potential benefits of prompt management,18,19 84%–95% of cases are not detected by attending physicians.3,7
These findings suggest that interventions to detect and manage delirium should be evaluated. In a previous trial conducted by our group the beneficial effects of systematic detection and management of delirium on cognition, behaviour and function were statistically significant but small.20 However, the design of this earlier study may have reduced the magnitude of treatment effects. Patients with mild delirium and those in whom delirium developed during their hospital stay (incident cases) were not included, enrolled patients were very old (mean age 86.1 years), the intervention nurse visited patients only 2 times per week, the sample of analysis was relatively small (n = 88), and the outcome measures (Short Portable Mental Status Questionnaire21 and Crichton Geriatric Behavioural Rating Scale) may have been insufficiently sensitive to the intervention effect. We therefore conducted a second trial to address the limitations of the previous study and to determine whether systematic detection and multidisciplinary care of delirium in older patients admitted to a general medical unit could reduce time to improvement in cognitive status. The secondary objectives of this trial were to determine whether the intervention could (a) reduce symptoms of delirium, increase independence in personal care activities, and improve the rate of discharge to the community, living arrangements after discharge and survival up to 8 weeks after enrolment; (b) decrease the length of hospital stay; and (c) improve the outcomes of some groups of patients more than others (i.e., those with incident v. prevalent delirium, those without v. with dementia, and those with less v. more comorbidity).
Methods
The study was conducted at St. Mary's Hospital, Montreal, a 400-bed university-affiliated primary acute care facility. All patients aged 65 or more admitted to the 5 general medical units between Mar. 15, 1996, and Jan. 31, 1999, were eligible. Excluded were patients who met 1 or more of the following exclusion criteria: primary diagnosis of stroke, duration of stay on the intensive care unit or cardiac monitoring unit of more than 48 hours, admission to geriatric or oncology service, inability to speak English or French, or residence other than on the island of Montreal.
To detect prevalent cases of delirium, eligible patients were screened within 24 hours after admission by the study nurse using the Short Portable Mental Status Questionnaire. Those who scored 3 to 9 errors on this instrument or had symptoms of delirium recorded in the nursing notes were assessed by means of the Confusion Assessment Method.22 This is a structured instrument that allows standardized recording of the 9 symptom domains of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders, third revised edition2 (DSM-III-R). To detect incident cases of delirium, all patients without prevalent delirium were rescreened during the week following admission. Those who scored 1 point higher on the Short Portable Mental Status Questionnaire than on admission or had symptoms of delirium recorded in the nursing notes were assessed with the Confusion Assessment Method. Patients with prevalent or incident delirium (criteria of the DSM-III-R2) were enrolled in the study.
Following enrolment, informed consent was obtained from the patient or substitute decision-maker. Patients were randomly allocated by means of computer-generated random numbers to receive the intervention or usual care on the 5 medical units. Stratified randomization was used; that is, independent randomization was done within groups of prevalent and incident cases respectively. Furthermore, we performed blocking using blocks of different sizes to guarantee similar numbers of patients in the control and intervention groups at any point and to ensure that the intervention team was not overloaded by a large number of patients during any period. Unequal block size also helped to maintain blinding as to treatment allocation. The study statistician prepared two series of sealed envelopes, one for each stratum, containing the treatment allocation. Following enrolment, the study nurse wrote the name of the patient on the appropriate envelope, then opened it to determine group allocation. If the patient was allocated to the intervention group, the study nurse started the intervention.
It was not feasible to randomly assign patients to intervention or control units in our hospital; however, in the previous trial, there was no evidence of contamination when patients in the 2 groups were managed on the same unit.20 Patients were asked for consent to participate in the study, and a close family member was asked for informed consent. The trial was approved by the hospital's Research Ethics Committee.
The intervention consisted of 2 parts: consultation and follow-up by a geriatric internist or psychiatrist, and follow-up in hospital by the study nurse. The consultation (within 24 hours after enrolment) determined the probable predisposing, precipitating and perpetuating factors of delirium (focusing on crucial factors associated with delirium, such as medication, infection and sensory deficits) and resulted in management recommendations (e.g., changes in medications and investigations to be carried out), which were recorded on a regular hospital consultation form and signalled in the progress notes. The follow-up by the study nurse involved daily visits (mean duration 35.7 minutes [standard deviation 28.8]) to conduct a brief structured mental status exam, monitor the completeness of the consultant's reports, ensure that previous recommendations had been implemented, ensure implementation of the nursing intervention protocol (Table 1) by liaising with the primary care nurses, and meet with the patient's family to involve them in patient care.
Table 1
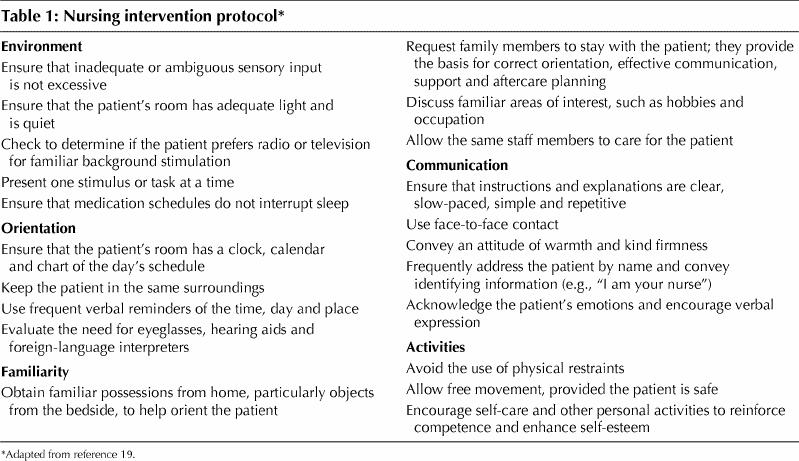
The intervention in the current study was more intensive than that in the previous trial20 in 4 ways. First, the consultant not only assessed but also followed the patients as required. Second, the study nurse visited the patient 5 days per week. Third, the intervention team (comprising 2 geriatric psychiatrists, 2 geriatric internists and the study nurse) met after every 8–10 patients were enrolled in the intervention group to discuss delirium management problems. Finally, the primary investigator met weekly with the study nurse to discuss problems of diagnosis, enrolment and interventions.
Usual care consisted of standard hospital services. Referrals (by attending physicians) for geriatric or psychiatric consultation were honoured consistent with usual practice, but patients in the usual care group did not receive systematic consultation by the geriatric specialists, follow-up by the study nurse or the nursing intervention protocol. Hospital staff were not informed of the diagnosis of delirium. We felt this approach was justified, for 2 reasons. First, patients were receiving care equivalent to the standard of care in most Canadian hospitals and, second, there is no evidence that detection of delirium and disclosure of the diagnosis to other caregivers is beneficial.
Within 24 hours after enrolment a research assistant, blinded as to treatment allocation, administered a baseline Mini-Mental Status Exam (MMSE),23 the Delirium Index24 and the Barthel Index.25 The MMSE measures cognitive function on a scale of 0 (poor) to 30 (excellent), a score of 23 or less indicating cognitive impairment. The Delirium Index measures the severity of delirium; the total score ranges from 0 (no symptoms) to 21 (severe symptoms). The Barthel Index measures independence in personal care activities; we used a modified scoring system that ranged from 0 (dependent) to 100 (independent).26 Subsequently, patients were evaluated 3 times (every other day) during the first week and weekly thereafter until discharge or 8 weeks in hospital.
Information related to length of hospital stay, survival within 8 weeks after enrolment and living arrangements after discharge was obtained from the hospital database and from follow-up interviews with informants. Living arrangements were ordered hierarchically from least dependent (e.g., home alone) to most dependent (e.g., nursing home); living arrangements at discharge were compared with those at admission and were rated as more dependent, same or less dependent.
Processes of care involved documentation of delirium by attending physicians, medication changes, consultations to occupational therapy, recreational therapy or social work, personal possessions at the bedside and nursing documentation of emotional support or orientation. To reflect processes of care, a present state checklist was completed within 24 hours after enrolment by the research assistant. Details of the methods and results of this process-of-care assessment are found in Appendix 1 (available online at www.cmaj.ca).
On enrolment the study nurse rated the overall clinical severity of illness27 and collected demographic data (including living arrangements before admission). Clinical severity of illness was rated on a scale from 1 (mild) to 9 (moribund). A family member was interviewed by the research assistant to complete the short form of the Informant Questionnaire on Cognitive Decline in the Elderly.28 This instrument assesses the possible presence of dementia before admission based on the responses of an informant who had known the patient for at least 5 years and could assess any decline in memory or cognition; the score is an average of the 16 item scores, each rated from 1 (much improved) to 5 (much worse). Patients with a mean score of 3.51 or more were considered to have dementia. After discharge (or 8 weeks in hospital) medical records were reviewed by a nurse-abstractor to complete the Charlson Comorbidity Index29 and living arrangements after discharge. The Charlson Comorbidity Index measures the number and severity of coexisting medical conditions, higher scores indicating more comorbidity.
The interrater agreement for delirium versus no delirium using the Confusion Assessment Method was excellent (kappa value 1.0) (n = 14); the study nurse's diagnosis of delirium had a sensitivity of 0.89 (n = 87) and a specificity of 1.0 compared with a consensus diagnosis.30 The interrater concordance correlation coefficient for the MMSE was 0.99 (n = 28).
To check on the extent of blinding of the research assistant during the study, the assistant was asked to guess which study group a convenience sample of 18 patients (whom the research assistant had recently assessed) belonged to. The research assistant's responses were correct for 2 of 4 patients in the intervention group and 3 of 5 patients in the usual care group; the assistant responded “don't know” for the 9 remaining patients (4 in the intervention group and 5 in the usual care group).
Characteristics of the patients at admission were compared between the study groups. Analyses of intervention effectiveness followed the intention-to-treat principle. The primary analysis was performed on a constructed binary outcome: “improved” or “not improved” during the hospital stay. Improvement in cognitive status was defined a priori as an increase in the MMSE score of 2 or more points (judged to be the minimum clinically significant change) compared to baseline, and no decrease below baseline plus 2 points in subsequent assessments. If the baseline MMSE score was 27 or more, improvement was defined as no decrease in the MMSE score below 27 in subsequent assessments. A patient who died was classified in the “not improved” category. The time to improvement was defined as the number of days between enrolment and the first assessment satisfying the definition of improvement. We used the Cox proportional hazards model31 to compare the intervention and usual care groups. The data were considered censored in the analysis for patients who had not improved at the time of discharge or withdrawal or 8 weeks after enrolment. In this context, a hazards ratio (HR) greater than 1 means a shorter time to improvement in the intervention group. In the secondary analyses, the interaction of the intervention with dementia, with comorbidity (Charlson Comorbidity Index < 3 v. ≥ 3) and with prevalent versus incident delirium were evaluated in separate Cox proportional hazards models because of the high level of multicolinearity between the covariates. Because the intervention was not expected to affect death, in secondary analyses we excluded deaths from the “not improved” category and considered deaths as censored.
To examine possible contamination effects, we divided the enrolment period into 3 equal time periods; the main analysis of time to improvement for the MMSE was repeated with the effects of time period and interaction of time period by intervention added to the Cox proportional hazards models used. Finally, we conducted an efficacy analysis in which we excluded patients in the intervention group who did not receive a consultation and those in the usual care group who did receive a consultation.32
We applied the same analyses for the secondary outcomes, the Delirium Index and the Barthel Index. The level of significance was set at 0.05. Confidence intervals (CIs) for the HRs were computed at the 95% level.
Results
The flow of patients through the study is shown in Fig. 1. The prevalence of delirium at admission was 12.6% (243/1925); the incidence among those without prevalent delirium was 3.3% (56/1682). There were no deviations from the planned study protocol.
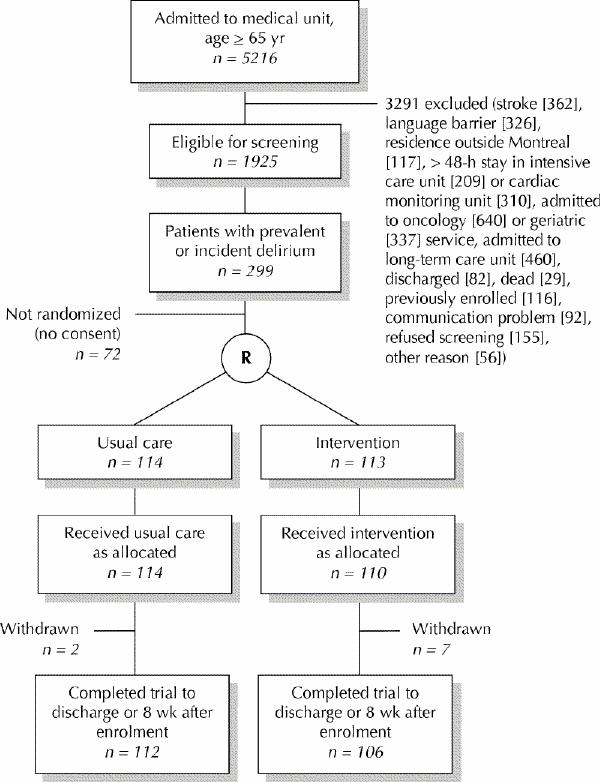
Fig. 1: Flow of patients through trial. R = randomization.
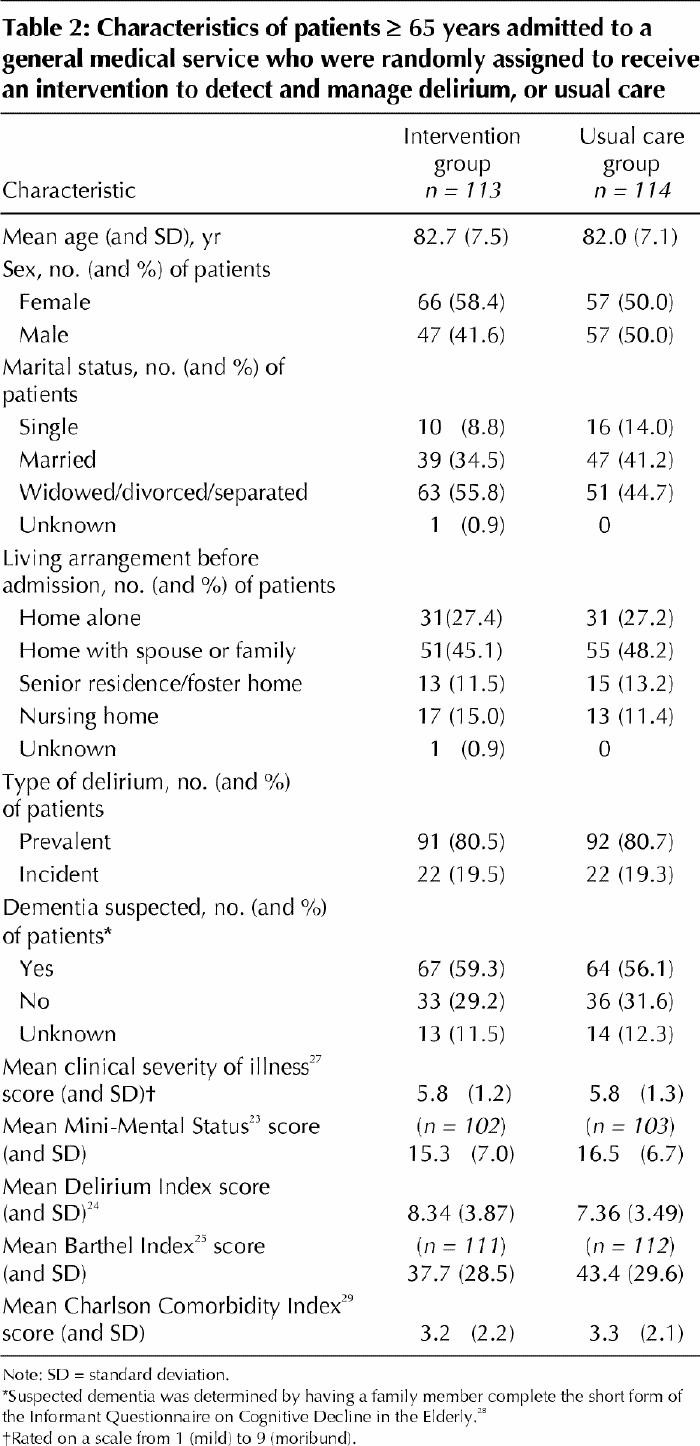
A total of 113 patients were allocated to the intervention group and 114 to the usual care group. At randomization, there were no clinically significant differences between the 2 groups in mean age, dementia status, or mean MMSE or Charlson Comorbidity Index score (Table 2). There were potentially clinically significant differences between the groups in sex (p = 0.2)and marital status (p = 0.19) (Table 2).
Table 2
The curve estimates of time to improvement in cognitive status (as determined with the MMSE), based on the Cox proportional hazards model, for the intervention and usual care groups are shown in Fig. 2. The rate of improvement at 36 days was similar in the 2 groups, but there was a nonsignificant trend toward a shorter time to improvement in the intervention group. The Cox proportional HR of intervention versus usual care, adjusted for age, sex and marital status, was 1.10 (95% CI 0.74–1.63). The proportion of patients who met the criteria for improvement was 48% in the intervention group and 45% in the usual care group. The results were similar when deaths were considered as censored rather than “not improved” and when patients who withdrew were excluded from the analysis.
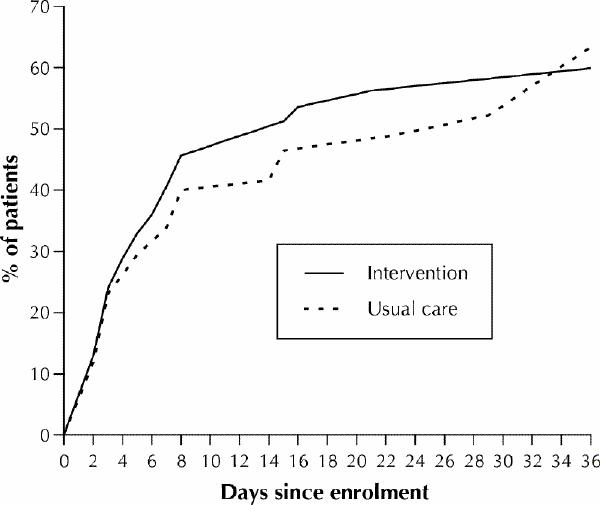
Fig. 2: Curve estimates of time to improvement in cognitive status, as determined with the Mini-Mental Status Exam,23 for the intervention group and the usual care group. The figure does not extend to 8 weeks because there were no new events after 36 days.
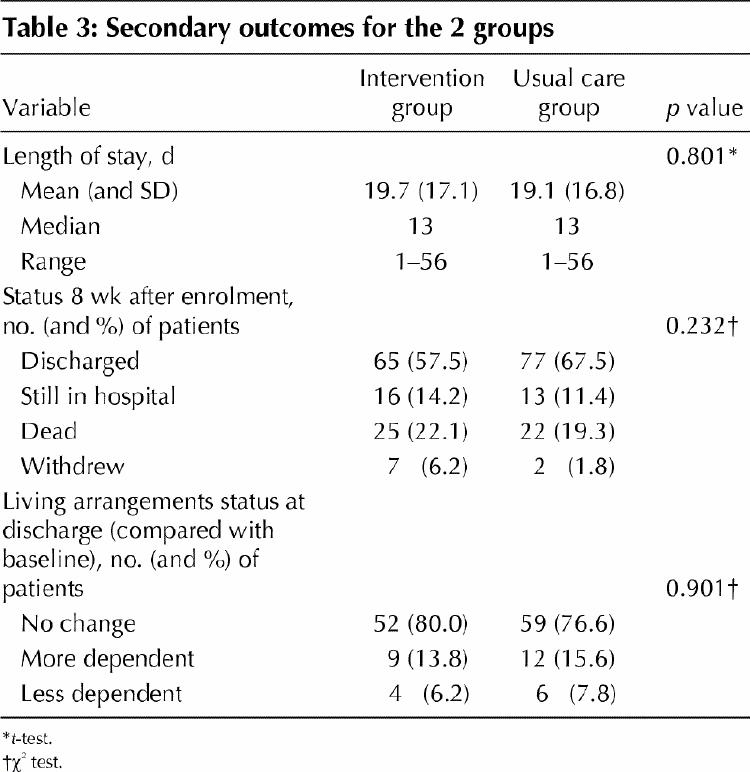
In the secondary analyses, there were no significant differences within 8 weeks after enrolment between the 2 study groups in time to improvement and rate of improvement in the Delirium Index score (HR 1.09, 95% CI 0.74–1.60) and the Barthel Index score, length of stay, rate of discharge to the community, living arrangements after discharge or survival (Table 3). There were no statistically significant differences between the 2 study groups in outcomes for patients who had no dementia (HR 1.54, 95% CI 0.80–2.97), those with less comorbidity (HR 1.36, 95% CI 0.75–2.46) or those with prevalent delirium (HR 1.15, 95% CI 0.48–2.79). As to contamination effects, there was no significant difference between the times to improvement for the MMSE during any of the 3 enrolment time periods. As well, the results of the efficacy analysis did not differ from the main analyses.
Table 3
Interpretation
In this randomized clinical trial we examined the effectiveness of systematic detection and multidisciplinary care of delirium in older patients admitted to a general medical service. Overall, the benefits of the intervention in terms of reducing the time to improvement in cognitive status were modest and not statistically significant. The primary analysis had 80% power to detect an HR of 1.65 at the 0.05 level with a 1-sided test. Subgroup analyses suggested that patients without dementia may be a subgroup that would benefit from the intervention, but our study lacked power to detect statistically significant differences in subgroups.
Eight features of this trial may have reduced the magnitude of intervention effects. First, in many cases the delirium may have been too mild or transient for the patient to have benefited from the intervention; however, subgroup analysis did not show that patients with severe delirium benefited more than those with mild delirium. Second, the intervention involved consultation by a geriatric specialist with follow-up as required; however, rates of compliance with the consultant's recommendations were relatively high, and we were able to demonstrate differences in the process of care by attending physicians. Third, the intervention nurse spent considerable time with the primary care nurses and families (in addition to patients); nonetheless, it appears that she was able to effect only modest changes in the process of nursing care, inasmuch as we were able to measure it. Fourth, although 3 patients in the treatment group did not receive the intervention and 21 patients in the control group received a consultation, the results of an efficacy analysis did not differ from the results of the primary analyses. Fifth, many patients had mild delirium superimposed on dementia. Our subgroup analyses suggested that subjects with dementia responded less to the intervention than did those without dementia, but the study lacked power to detect such effects in subgroups. Sixth, a Hawthorne effect, whereby implementation of the study resulted in improved detection and management of all patients with delirium, may have reduced the observed differences between the treatment and control groups. Documentation of delirium by attending physicians in subjects receiving usual care was higher than generally reported (27% v. 10%–15%3,7), but the rate of improvement in the usual care group (45%) was similar to or lower than the rates of improvement reported in descriptive studies.4,8,10 Seventh, because patients receiving the intervention and usual care were managed on the same units by the same nurses, the care of the patients in the control group may have been contaminated by the intervention program; however, there was no evidence of an intervention–time period interaction effect for the MMSE. Finally, the standard of usual care in our hospital may have been too good to allow detection of a difference between usual and enhanced care; however, data on the process of care in the usual care group did not support this contention.
Two other factors may have contributed to the negative results. First, our intervention did not include a specific (neurochemical) treatment for delirium because none exists. Second, delirium may be an epiphenomenon related to the severity of medical illness; consequently, the psychosocial component of the intervention may have been superfluous.
Our results have implications for clinical practice and research. Systematic detection and multidisciplinary care of delirium in older patients admitted to a general medical unit does not appear to be more effective than usual hospital care that focuses on the diagnosis and management of the medical conditions contributing to the delirium. The additional effort to detect delirium early in the hospital stay of these patients and the substantial time required to provide enhanced care do not seem to improve outcomes and may be unwarranted. In addition, the level of medical monitoring and intervention in an acute care hospital medical setting may be too intensive to detect any added effect of systematic detection and multidisciplinary care of delirium, but such intervention in settings providing a lower level of care (e.g., chronic care hospitals, nursing homes) may be effective. The process-of-care analysis suggests that the intervention was able to change the process of care of elderly delirious patients by physicians, families and, to a lesser extent, ward nurses. Finally, the management protocol for delirium in this study was similar to the practice guidelines of the American Psychiatric Association:18 clearly we need better treatment guidelines for delirium.
Because our intervention program was in part consultative in nature, some aspects of recommended care were not fully implemented, particularly by nursing staff. A fully implemented intervention may have resulted in more substantial outcomes. It is possible that a similar intervention may be effective if administered in the context of direct care on units where physicians and nursing staff are more attuned to the special needs of older patients (e.g., geriatric units).
Finally, in the absence of an effective intervention strategy for prevalent or incident delirium in older patients admitted to a medical service, research efforts should focus on the prevention of delirium in this population. Effective programs to prevent incident delirium in elderly patients on medical33 and surgical34 units (including those with dementia) have been described, but programs to prevent prevalent delirium in older patients admitted to a medical unit have yet to be developed. This development might involve the identification of potentially modifiable predisposing or precipitating risk factors for prevalent delirium in community or nursing home populations (e.g., medication use) and evaluation of interventions aimed at risk factor abatement (e.g., medication management).
β See related article page 763
Acknowledgments
We gratefully acknowledge the collaboration and contributions of Eric Belzile and Michael Abrahamowicz and the medical and nursing staff of St. Mary's Hospital.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Cole, McCusker and Bellavance designed the study, secured funding, executed the study, analyzed and interpreted the data and drafted the article. Drs. Primeau, Bailey and Bonnycastle and Ms. Laplante participated in the acquisition of data and revision of the article. All authors approved the final version of the manuscript.
This study was funded by grant 6605-4686-403 from the National Health Research Development Program of Health Canada.
Competing interests: None declared for Drs. Cole, McCusker, Bellavance, Bailey and Bonnycastle and Ms. Laplante. Dr. Primeau has received speaker fees from Janssen-Ortho Inc., Wyeth–Ayerst Canada Inc. and Pfizer Canada Inc.
Correspondence to: Dr. Martin G. Cole, Department of Psychiatry, St. Mary's Hospital, 3830 Lacombe Ave., Montreal QC H3T 1M5; fax 514 734-2652
References
- 1.Lipowski Z. Delirium: acute confusional states. New York: Oxford University Press; 1990.
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd rev ed. Washington: The Association; 1987. p. 103.
- 3.Thomas RI, Cameron DJ, Fahs MC. A prospective study of delirium and prolonged hospital stay: exploratory study. Arch Gen Psychiatry 1988;45: 937-40. [DOI] [PubMed]
- 4.Rockwood K. The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol 1993;48(4):M162-6. [DOI] [PubMed]
- 5.Chisholm S, Deniston L, Igrisan R, Barbus A. Prevalence of confusion in elderly hospitalized patients. J Gerontol Nurs 1982;8(2):87-96. [DOI] [PubMed]
- 6.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA 1990;263(8):1097-101. [PubMed]
- 7.Johnson JC, Gottlieb GL, Sullivan E, Wanich C, Kinosian B, Forciea MA, et al. Using DSM-III criteria to diagnose delirium in elderly general medical patients. J Gerontol 1990;45(3):M113-9. [DOI] [PubMed]
- 8.Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: a prospective study. Q J Med 1992;300:307-14. [PubMed]
- 9.Kolbeinsson H, Jonsson A. Delirium and dementia in acute medical admissions of elderly patients in Iceland. Acta Psychiatr Scand 1993;87:123-7. [DOI] [PubMed]
- 10.Levkoff SE, Evans DA, Liptzin B, Cleary PD, Lipsitz LA, Wetle TT, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992;152:334-40. [DOI] [PubMed]
- 11.Inouye S, Rushing J, Foreman M, Palmer R, Pompei P. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med 1998;13:234-42. [DOI] [PMC free article] [PubMed]
- 12.Cameron DJ, Thomas RI, Mulvihill M, Bronheim H. Delirium: a test of the Diagnostic and Statistical Manual III criteria on medical inpatients. J Am Geriatr Soc 1987;35(11):1007-10. [DOI] [PubMed]
- 13.Francis J, Kapoor WN. Prognosis after hospital discharge of older medical patients with delirium. J Am Geriatr Soc 1992;40(6):601-6. [DOI] [PubMed]
- 14.Lamont C, Sampson S, Matthias R, Kane R. The outcome of hospitalization for acute illness in the elderly. J Am Geriatr Soc 1983;31(5):282-8. [DOI] [PubMed]
- 15.Murray AM, Levkoff SE, Wetle TT, Beckett L, Cleary PD, Schor JD, et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol 1993;48(5):M181-6. [DOI] [PubMed]
- 16.Rabins PV, Folstein MF. Delirium and dementia: diagnostic criteria and fatality rates. Br J Psychiatry 1982;140:149-53. [DOI] [PubMed]
- 17.Cole M, Primeau F. Prognosis of delirium in elderly hospital patients. CMAJ 1993;149(1):41-6. [PMC free article] [PubMed]
- 18.Practice guidelines for treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry 1999;156(5 Suppl):1-20. [PubMed]
- 19.Beresin E. Delirium in the elderly. J Geriatr PsychiatryNeurol 1988;1:127-43. [DOI] [PubMed]
- 20.Cole MG, Primeau FJ, Bailey RF, Bonnycastle MJ, Masciarelli F, Engelsmann F, et al. Systematic intervention for elderly inpatients with delirium: a randomized trial. CMAJ 1994;151(7):965-70. [PMC free article] [PubMed]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23(10):433-41. [DOI] [PubMed]
- 22.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RJ. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. [DOI] [PubMed]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. [DOI] [PubMed]
- 24.McCusker J, Cole M, Bellavance F, Primeau F. Reliability and validity of a new measure of severity of delirium. Int Psychogeriatr 1998;10(4):421-33. [DOI] [PubMed]
- 25.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14(2):61-5. [PubMed]
- 26.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol 1989;42(8):703-9. [DOI] [PubMed]
- 27.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG Jr. Assessing illness severity: Does clinical judgment work? J Chronic Dis 1986; 39 (6): 439-52. [DOI] [PubMed]
- 28.Jorm A. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24:145-53. [DOI] [PubMed]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373-83. [DOI] [PubMed]
- 30.Zou Y, Cole MG, Primeau FJ, McCusker J, Bellavance F, Laplante J. Detection and diagnosis of delirium in the elderly: Psychiatrist diagnosis, confusion assessment method or consensus diagnosis? Int Psychogeriatr 1998;10 (3):303-8. [DOI] [PubMed]
- 31.Cox D, Oakes D. Analysis of survival data. London: Chapman and Hall; 1984. p. 91-109.
- 32.Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21(5):837-41. [DOI] [PubMed]
- 33.Inouye SK, Bogardus ST Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340(9):669-76. [DOI] [PubMed]
- 34.Cole M, Primeau F, Élie L. Delirium: prevention, treatment, and outcome studies. J Geriatr Psychiatry Neurol 1998;11:126-37. [DOI] [PubMed]


