Abstract
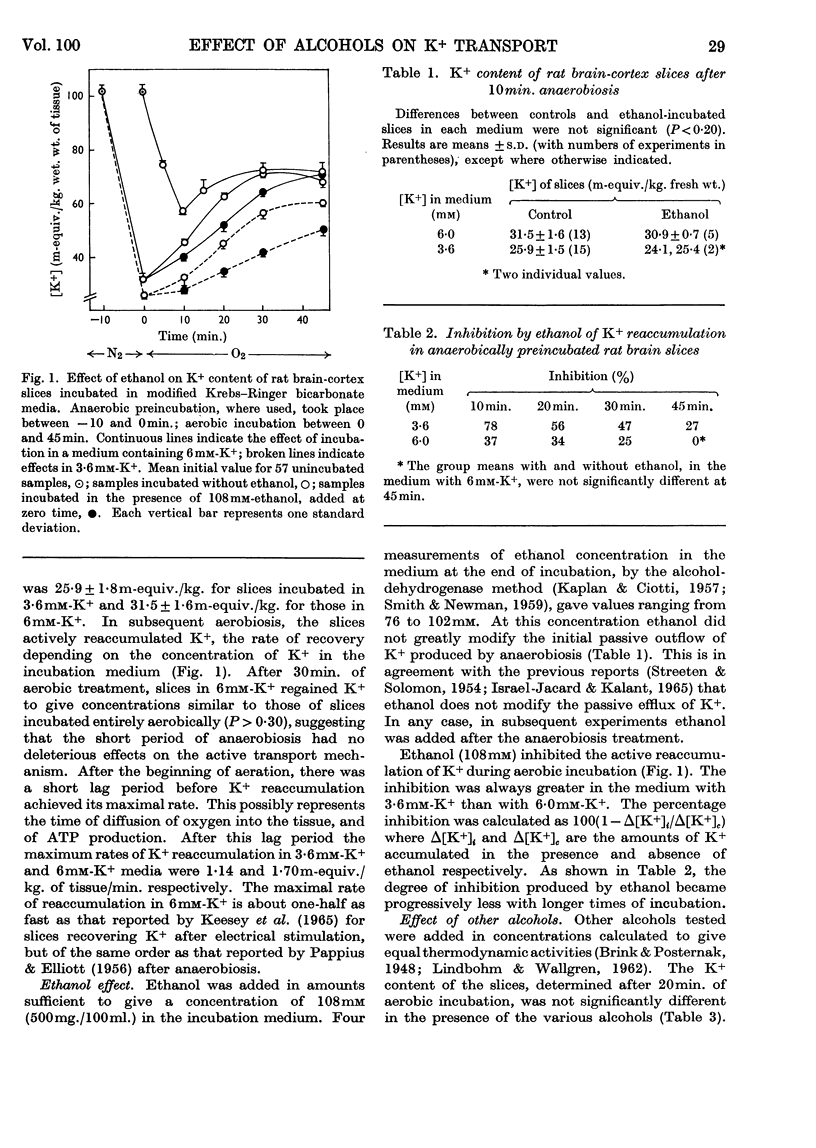
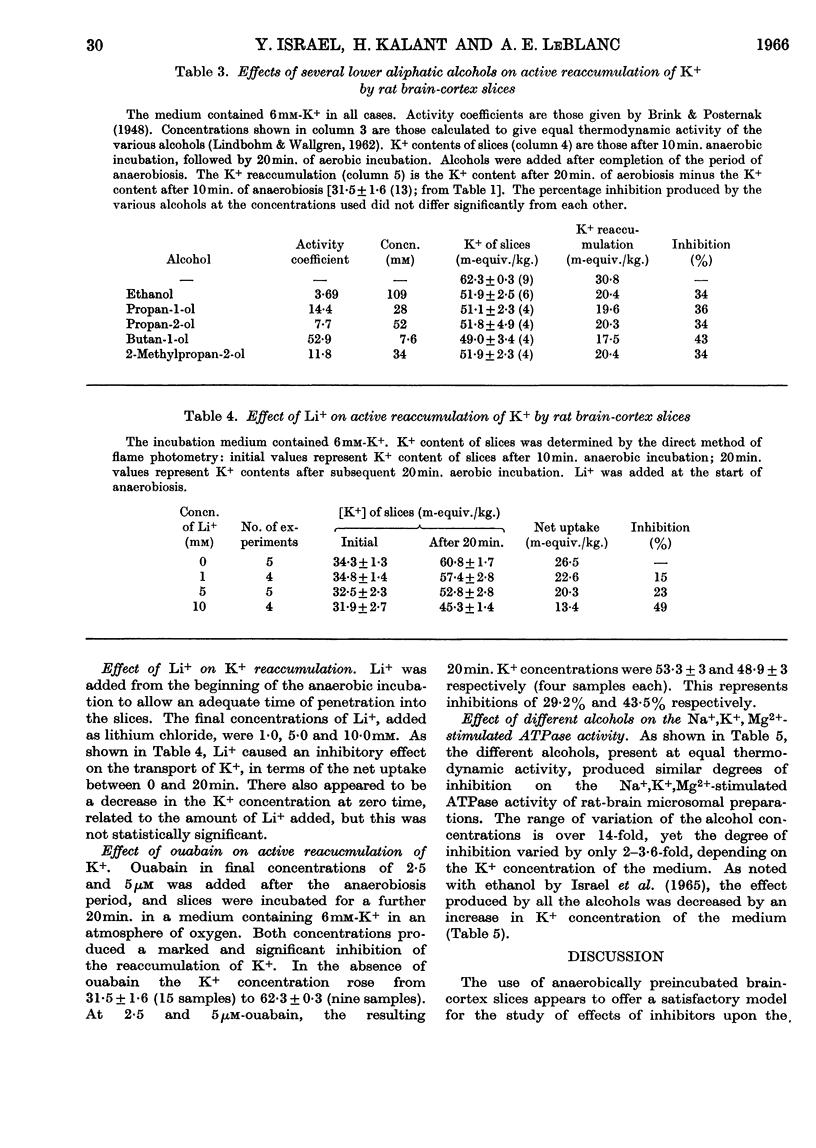
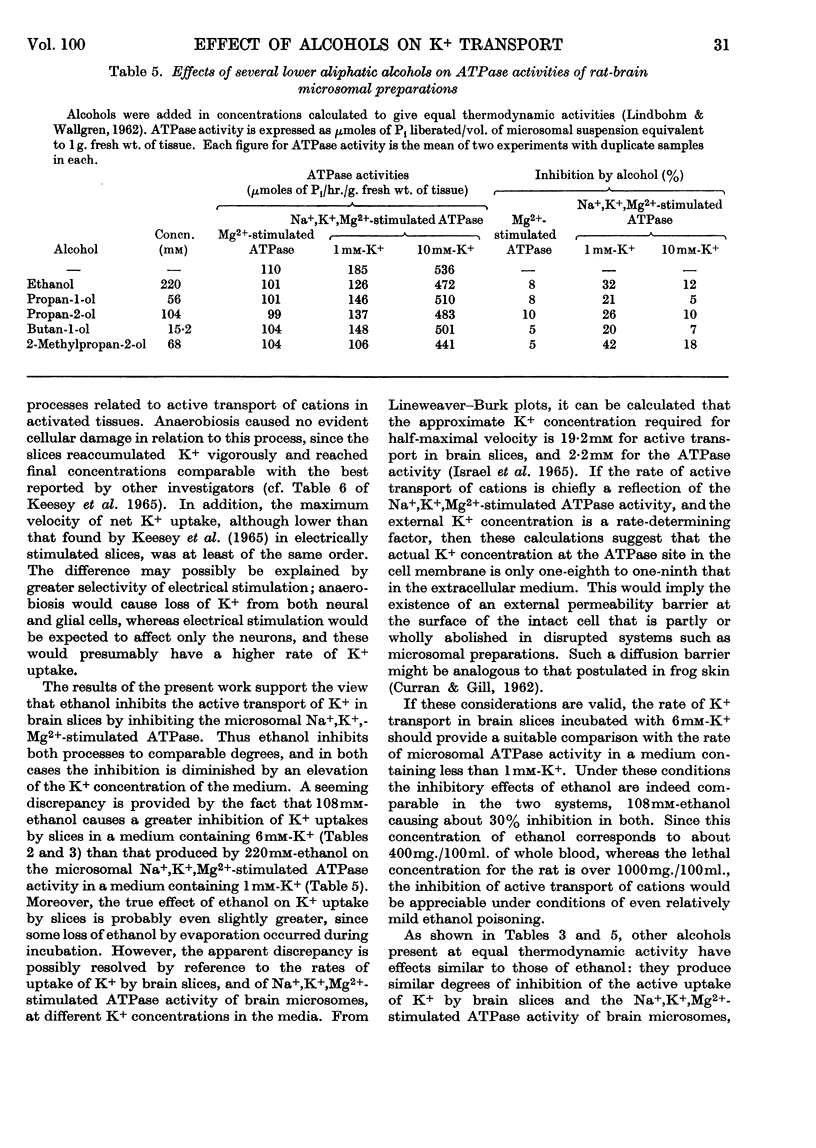
1. Slices of rat cerebral cortex, incubated anaerobically at 37°, lost K+ from an initial concentration of 102m-equiv./kg. to a concentration of 57m-equiv./kg. after 10min. On subsequent aerobic incubation they regained K+ rapidly at a rate that varied with the K+ concentration of the medium. 2. Lower aliphatic alcohols, present at equal thermodynamic activity, produced approximately equal degrees of inhibition of K+ uptake during the aerobic incubation. This inhibition was reduced by an increase in K+ content of the medium. Ethanol did not affect the rate of K+ loss during anaerobic incubation. 3. Li+, in concentrations of 1–10mm, also inhibited K+ uptake by brain-cortex slices, the degree of inhibition varying with the Li+ concentration. Ouabain also inhibited K+ uptake. 4. The same series of alcohols, at equal thermodynamic activity, produced comparable degrees of inhibition of Na+,K+,Mg2+-stimulated adenosine-triphosphatase activity in brain microsomes. 5. It is suggested that inhibition of cation transport is an important, but not a primary, mechanism in the production of central nervous depression by alcohols and other substances.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L. Sodium-potassium-activated adenosine triphosphatase in the squid giant axon. Nature. 1962 Jun 23;194:1180–1181. doi: 10.1038/1941180a0. [DOI] [PubMed] [Google Scholar]
- CHAN P. C., CALABRESE V., THEIL L. S. SPECIES DIFFERENCES IN THE EFFECT OF SODIUM AND POTASSIUM IONS ON THE ATPASE OF ERYTHROCYTE MEMBRANES. Biochim Biophys Acta. 1964 Mar 30;79:424–426. [PubMed] [Google Scholar]
- CURRAN P. F., GILL J. R., Jr The effect of calcium on sodium transport by frog skin. J Gen Physiol. 1962 Mar;45:625–641. doi: 10.1085/jgp.45.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT K. A., PAPPIUS H. M. Factors affecting the potassium content of incubated brain slices. Can J Biochem Physiol. 1956 Sep;34(5):1053–1067. [PubMed] [Google Scholar]
- ISRAEL-JACARD Y., KALANT H. EFFECT OF ETHANOL ON ELECTROLYTE TRANSPORT AND ELECTROGENESIS IN ANIMAL TISSUES. J Cell Physiol. 1965 Feb;65:127–132. doi: 10.1002/jcp.1030650115. [DOI] [PubMed] [Google Scholar]
- ISRAEL Y., KALANT H. EFFECT OF ETHANOL ON THE TRANSPORT OF SODIUM IN FROG SKIN. Nature. 1963 Nov 2;200:476–478. doi: 10.1038/200476a0. [DOI] [PubMed] [Google Scholar]
- Israel Y., Kalant H., Laufer I. Effects of ethanol on na, K, mg-stimulated microsomal ATPase activity. Biochem Pharmacol. 1965 Dec;14(12):1803–1814. doi: 10.1016/0006-2952(65)90270-4. [DOI] [PubMed] [Google Scholar]
- KEESEY J. C., WALLGREN H., MCILWAIN H. THE SODIUM, POTASSIUM AND CHLORIDE OF CEREBRAL TISSUES: MAINTENANCE, CHANGE ON STIMULATION AND SUBSEQUENT RECOVERY. Biochem J. 1965 May;95:289–300. doi: 10.1042/bj0950289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAF A. Some actions of neurohypophyseal hormones on a living membrane. J Gen Physiol. 1960 May;43:175–189. doi: 10.1085/jgp.43.5.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDBOHM R., WALLGREN H. Changes in respiration of rat brain cortex slices induced by some aliphatic alcohols. Acta Pharmacol Toxicol (Copenh) 1962;19:53–58. doi: 10.1111/j.1600-0773.1962.tb00339.x. [DOI] [PubMed] [Google Scholar]
- LYON A. F., DEGRAFF A. C. The neurotoxic effects of digitalis. Am Heart J. 1963 Jun;65:839–840. doi: 10.1016/0002-8703(63)90249-7. [DOI] [PubMed] [Google Scholar]
- MUDGE G. H. Studies on potassium accumulation by rabbit kidney slices; effect of metabolic activity. Am J Physiol. 1951 Apr 1;165(1):113–127. doi: 10.1152/ajplegacy.1951.165.1.113. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- Quastel J. H. Molecular transport at cell membranes. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):169–196. doi: 10.1098/rspb.1965.0065. [DOI] [PubMed] [Google Scholar]
- SCHOU M. Biology and pharmacology of the lithium ion. Pharmacol Rev. 1957 Mar;9(1):17–58. [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C., ZERAHN K. Investigations on the effect of some local anaesthetics and other amines on the active transport of sodium through the isolated short-circuited frog skin. Biochim Biophys Acta. 1959 Oct;35:324–333. doi: 10.1016/0006-3002(59)90381-6. [DOI] [PubMed] [Google Scholar]
- SMITH M. E., NEWMAN H. W. The rate of ethanol metabolism in fed and fasting animals. J Biol Chem. 1959 Jun;234(6):1544–1549. [PubMed] [Google Scholar]
- STREETEN D. H., SOLOMON A. K. The effect of ACTH and adrenal steroids on K transport in human erythrocytes. J Gen Physiol. 1954 May 20;37(5):643–661. doi: 10.1085/jgp.37.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLGREN H., KULONEN E. Effect of ethanol on respiration of rat-brain-cortex slices. Biochem J. 1960 Apr;75:150–158. doi: 10.1042/bj0750150. [DOI] [PMC free article] [PubMed] [Google Scholar]


