Abstract
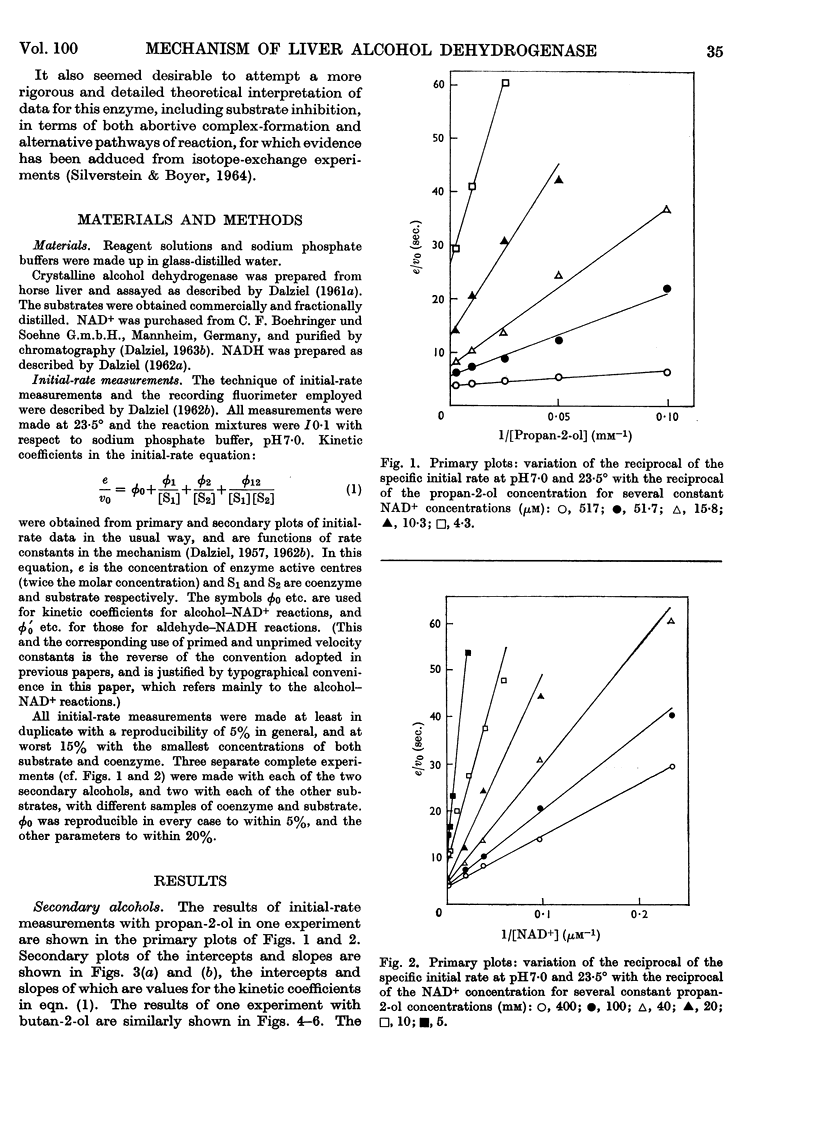
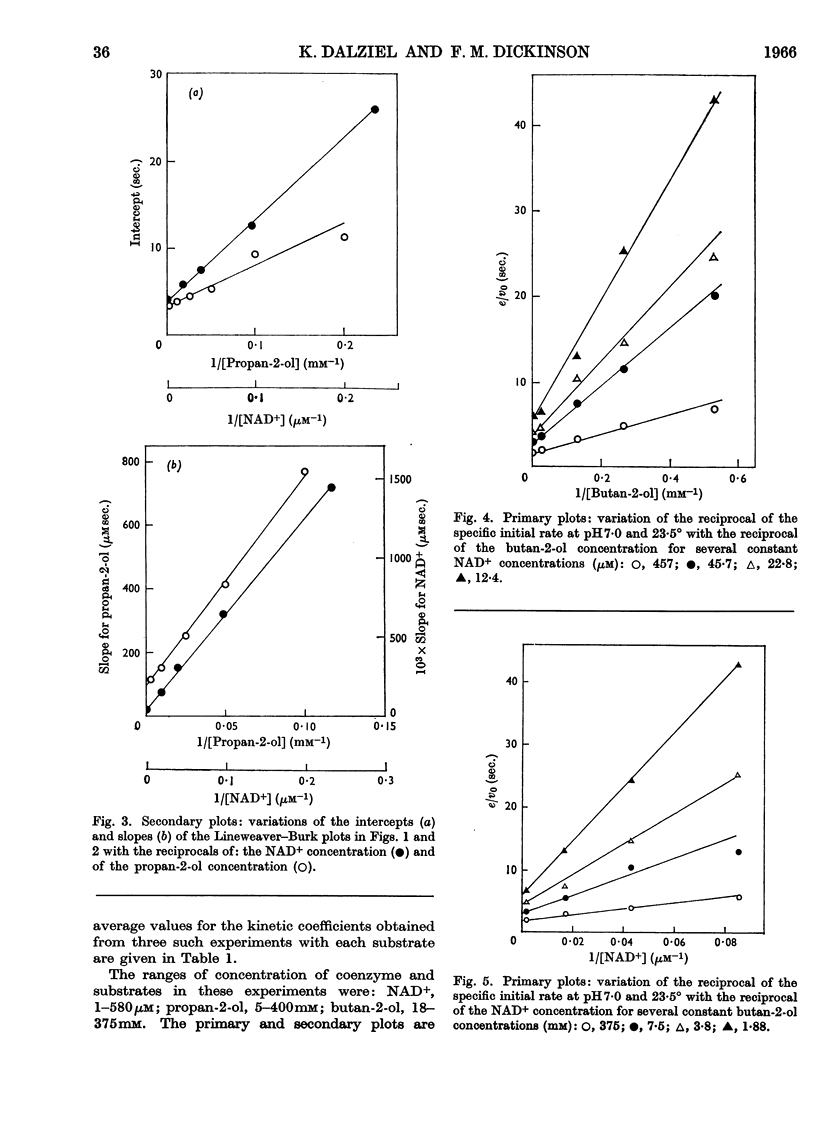
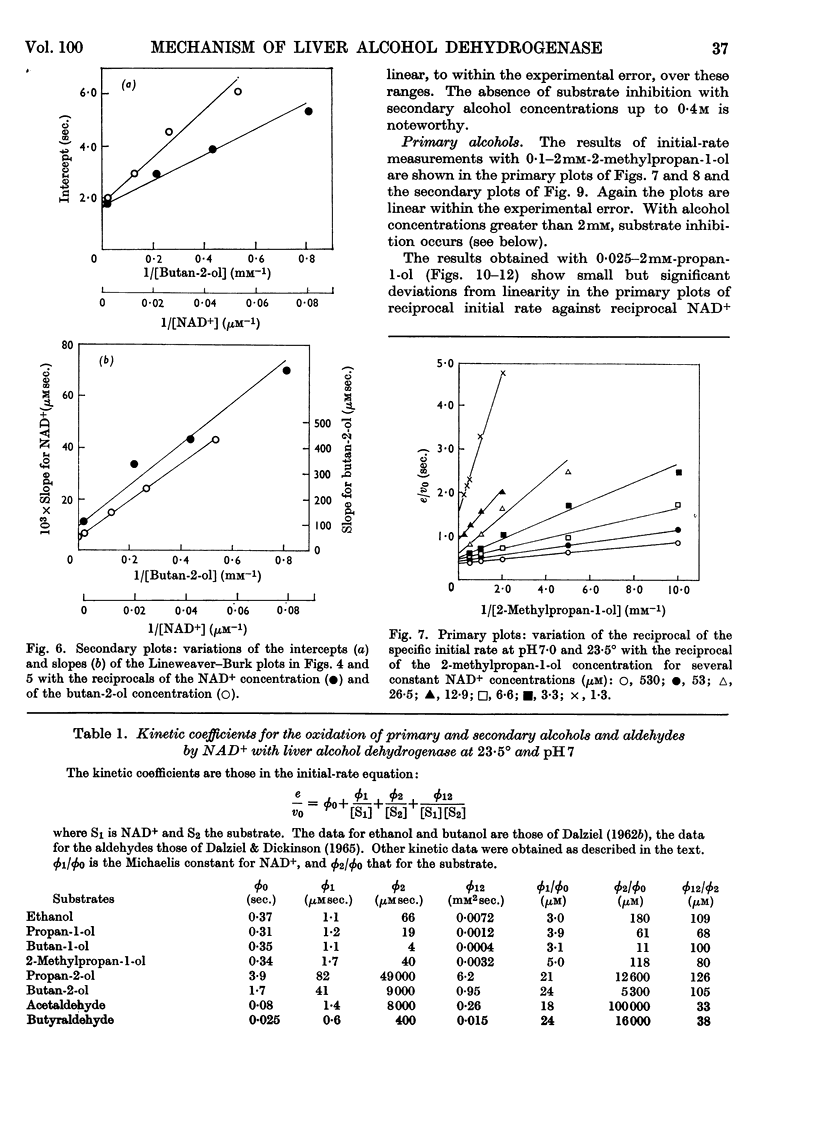
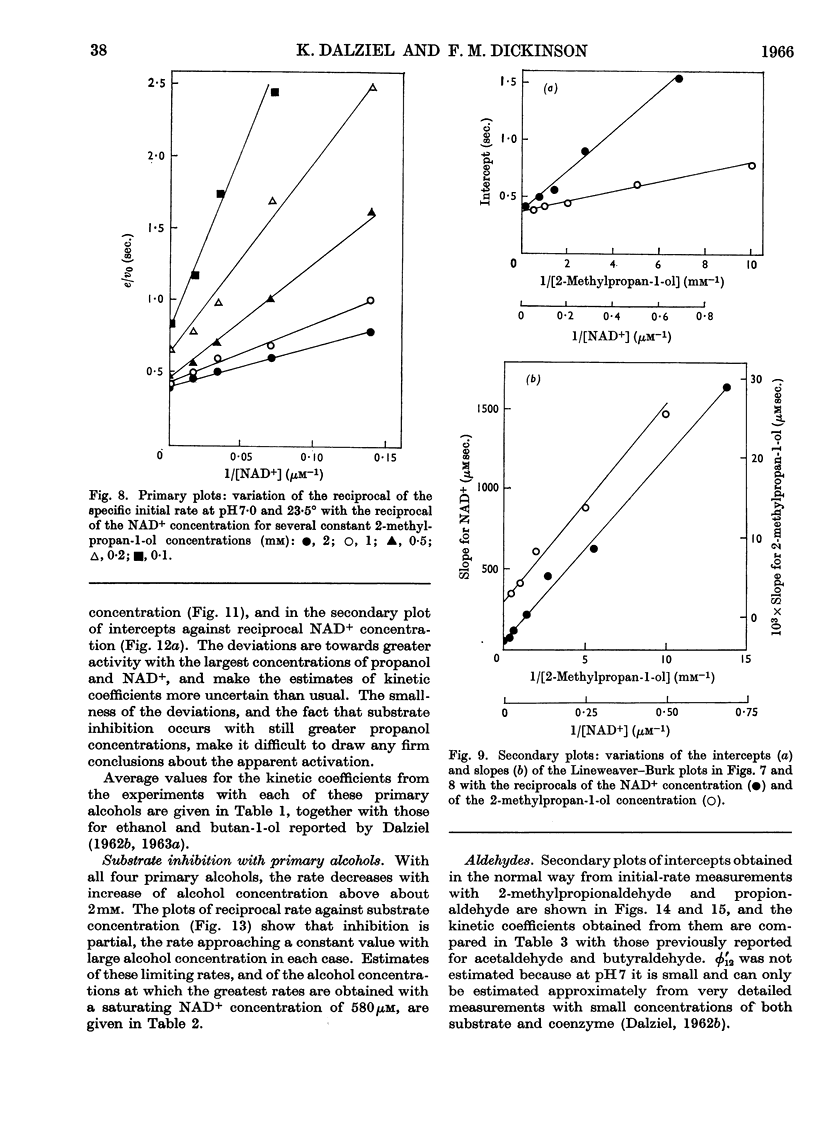
1. The activity of liver alcohol dehydrogenase with propan-2-ol and butan-2-ol has been confirmed. The activity with the corresponding ketones is small. Initial-rate parameters are reported for the oxidation of these secondary alcohols, and of propan-1-ol and 2-methylpropan-1-ol, and for the reduction of propionaldehyde and 2-methylpropionaldehyde. Substrate inhibition with primary alcohols is also described. 2. The requirements of the Theorell–Chance mechanism are satisfied by the data for all the primary alcohols and aldehydes, but not by the data for the secondary alcohols. A mechanism that provides for dissociation of either coenzyme or substrate from the reactive ternary complex is described, and shown to account for the initial-rate data for both primary and secondary alcohols, and for isotope-exchange results for the former. With primary alcohols, the rapid rate of reaction of the ternary complex, and its small steady-state concentration, result in conformity of initial-rate data to the requirements of the Theorell–Chance mechanisms. With secondary alcohols, the ternary complex reacts more slowly, its steady-state concentration is greater, and therefore dissociation of coenzyme from it is rate-limiting with non-saturating coenzyme concentrations. 3. Substrate inhibition with large concentrations of primary alcohols is attributed to the formation of an abortive complex of enzyme, NADH and alcohol from which NADH dissociates more slowly than from the enzyme–NADH complex. The initial-rate equation is derived for the complete mechanism, which includes a binary enzyme–alcohol complex and alternative pathways for formation of the reactive ternary complex. This mechanism would also provide, under suitable conditions, for substrate activation or substrate inhibition in a two-substrate reaction, according to the relative rates of reaction through the two pathways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The preparation and properties of crystalline alcohol dehydrogenase from liver. Biochem J. 1961 Aug;80:440–445. doi: 10.1042/bj0800440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. Aldehyde mutase. Nature. 1965 Apr 17;206(981):255–257. doi: 10.1038/206255a0. [DOI] [PubMed] [Google Scholar]
- KOSICKI G. W., SRERE P. A. Kinetic studies on the citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2560–2565. [PubMed] [Google Scholar]
- MAHLER H. R., BAKER R. H., Jr, SHINER V. J., Jr Studies on the mechanism of enzyme-catalyzed oxidation reduction reactions. IV. A proposed mechanism for the over-all reaction catalyzed by liver alcohol dehydrogenase. Biochemistry. 1962 Jan;1:47–52. doi: 10.1021/bi00907a008. [DOI] [PubMed] [Google Scholar]
- MASSEY V., VEEGER C. BIOLOGICAL OXIDATIONS. Annu Rev Biochem. 1963;32:579–638. doi: 10.1146/annurev.bi.32.070163.003051. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., STACHOW C. S., COOK R. A. A KINETIC MODEL FOR THE MECHANISM OF ALLOSTERIC ACTIVATION OF NICOTINAMIDE-ADENINE DINUCLEOTIDE-SPECIFIC ISOCRITIC DEHYDROGENASE. Biochemistry. 1965 Mar;4:410–421. doi: 10.1021/bi00879a006. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- THEORELL H., WINER A. D. Dissociation constants of the liver alcohol dehydrogenase coenzyme complexes. Arch Biochem Biophys. 1959 Jul;83(1):291–308. doi: 10.1016/0003-9861(59)90035-9. [DOI] [PubMed] [Google Scholar]
- VAN EYS J. Aldehyde-ketone isomerization activity of liver alcohol dehydrogenase. J Biol Chem. 1961 May;236:1531–1533. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Fluorescence spectra of ternary complexes of dehydrogenases with reduced diphosphopyridine nucleotide and reduced substrates. Biochim Biophys Acta. 1958 Aug;29(2):424–425. doi: 10.1016/0006-3002(58)90203-8. [DOI] [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. ISOTOPIC EXCHANGE AT EQUILIBRIUM AS A CRITERION OF ENZYMATIC MECHANISMS. Nature. 1964 Aug 1;203:492–494. doi: 10.1038/203492a0. [DOI] [PubMed] [Google Scholar]
- WRATTEN C. C., CLELAND W. W. PRODUCT INHIBITION STUDIES ON YEAST AND LIVER ALCOHOL DEHYDROGENASES. Biochemistry. 1963 Sep-Oct;2:935–941. doi: 10.1021/bi00905a007. [DOI] [PubMed] [Google Scholar]


