Abstract
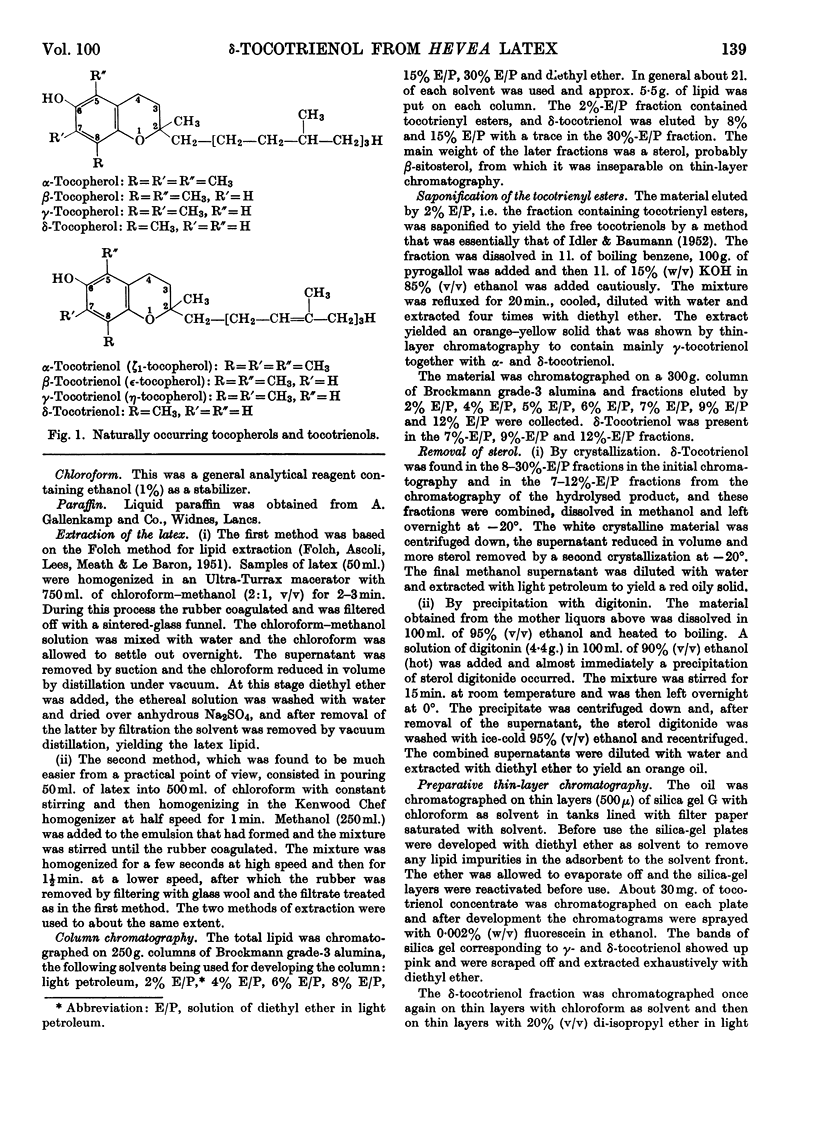
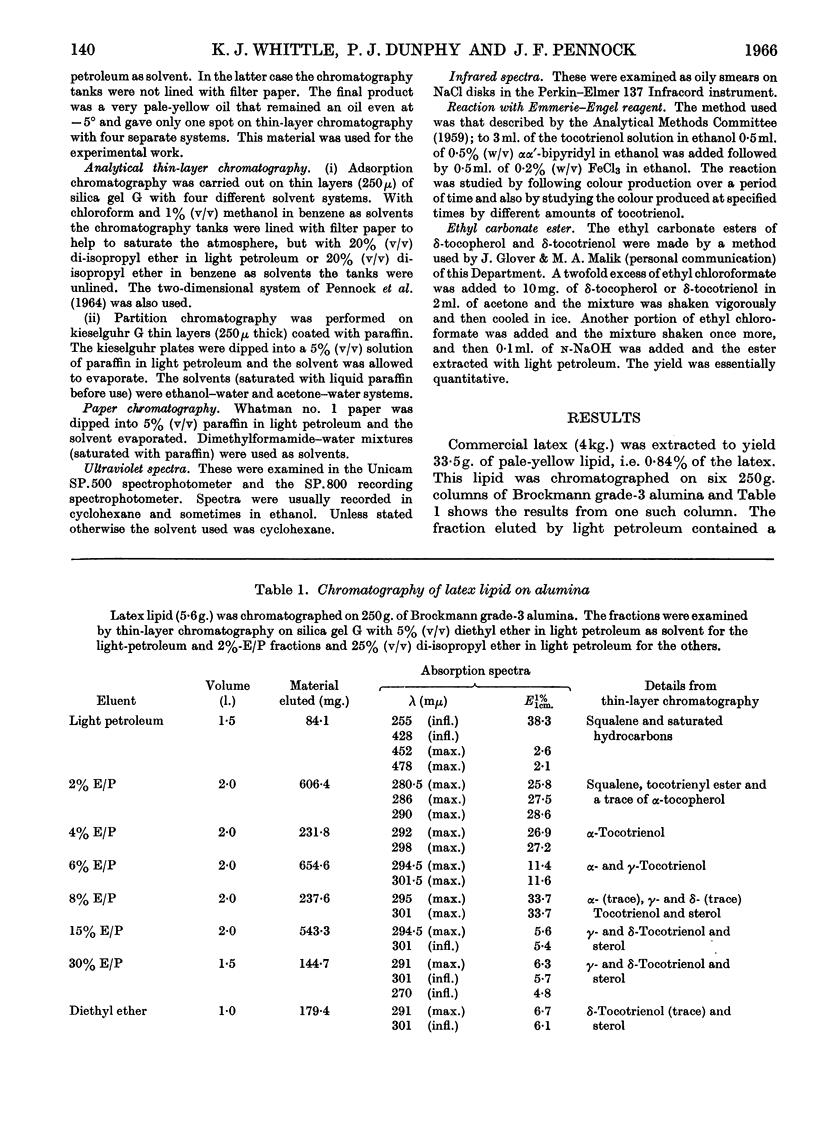
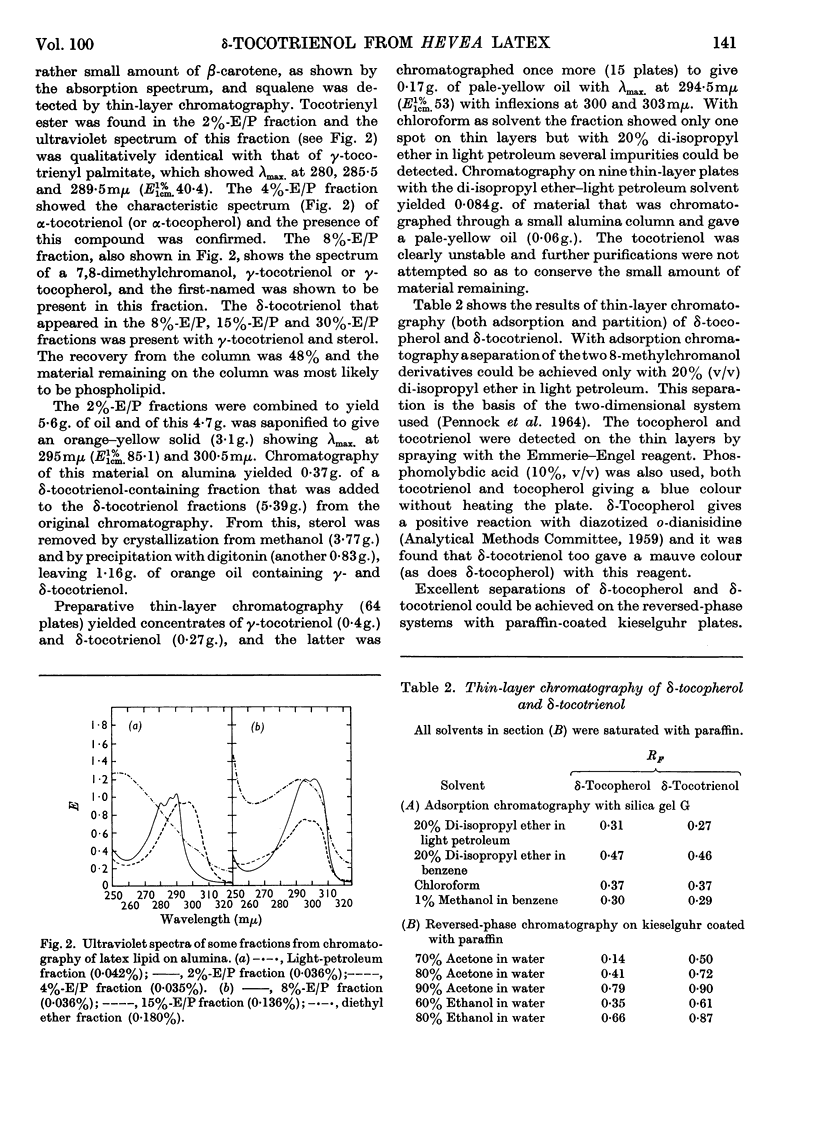
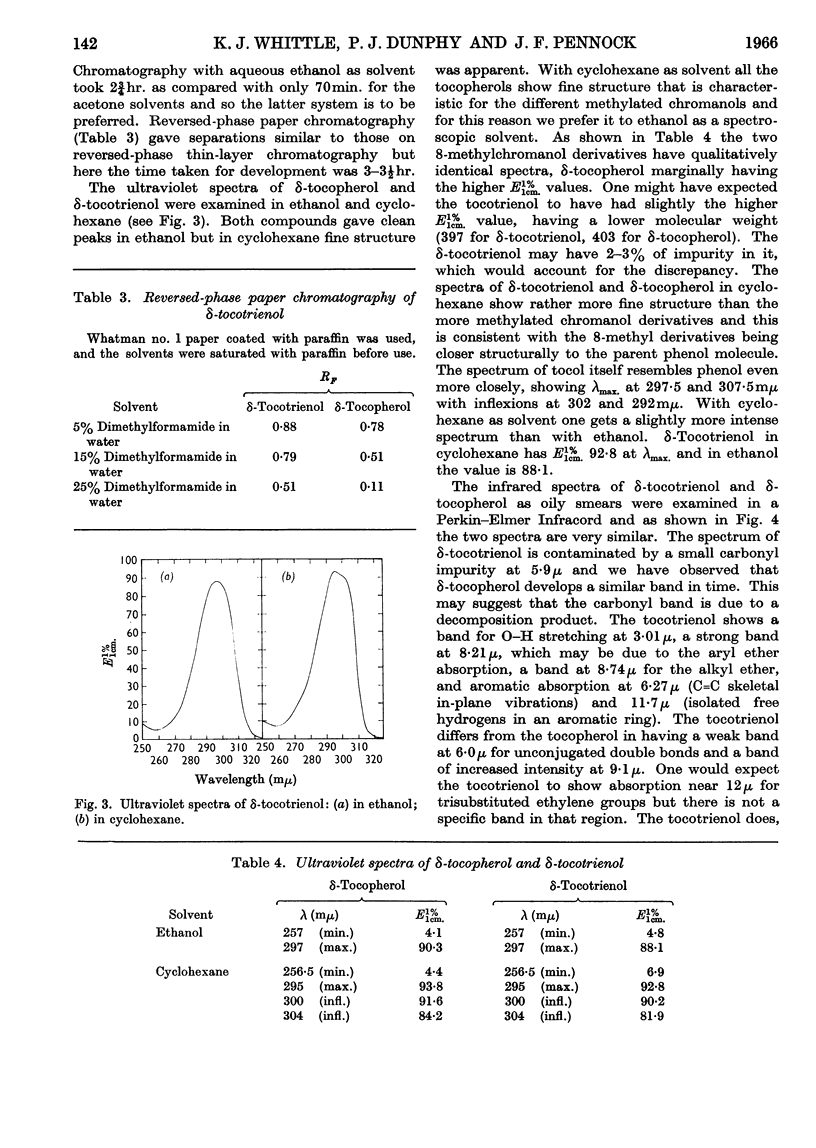
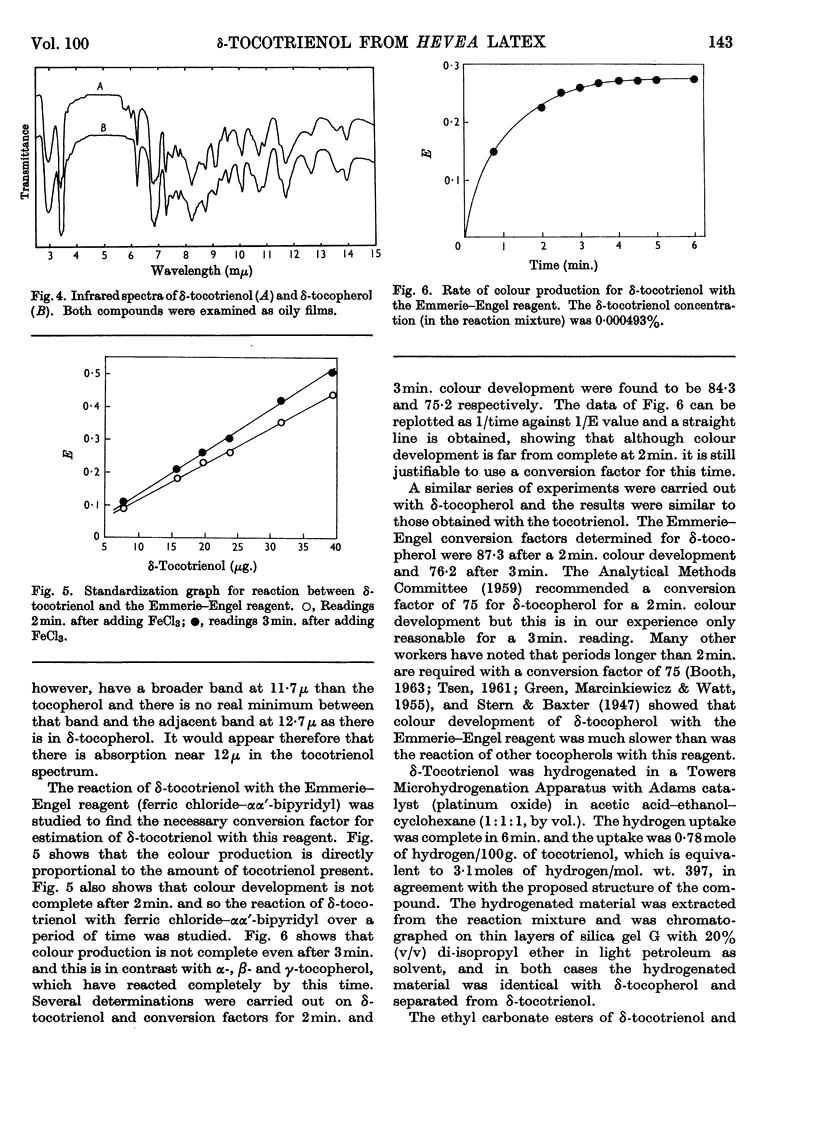
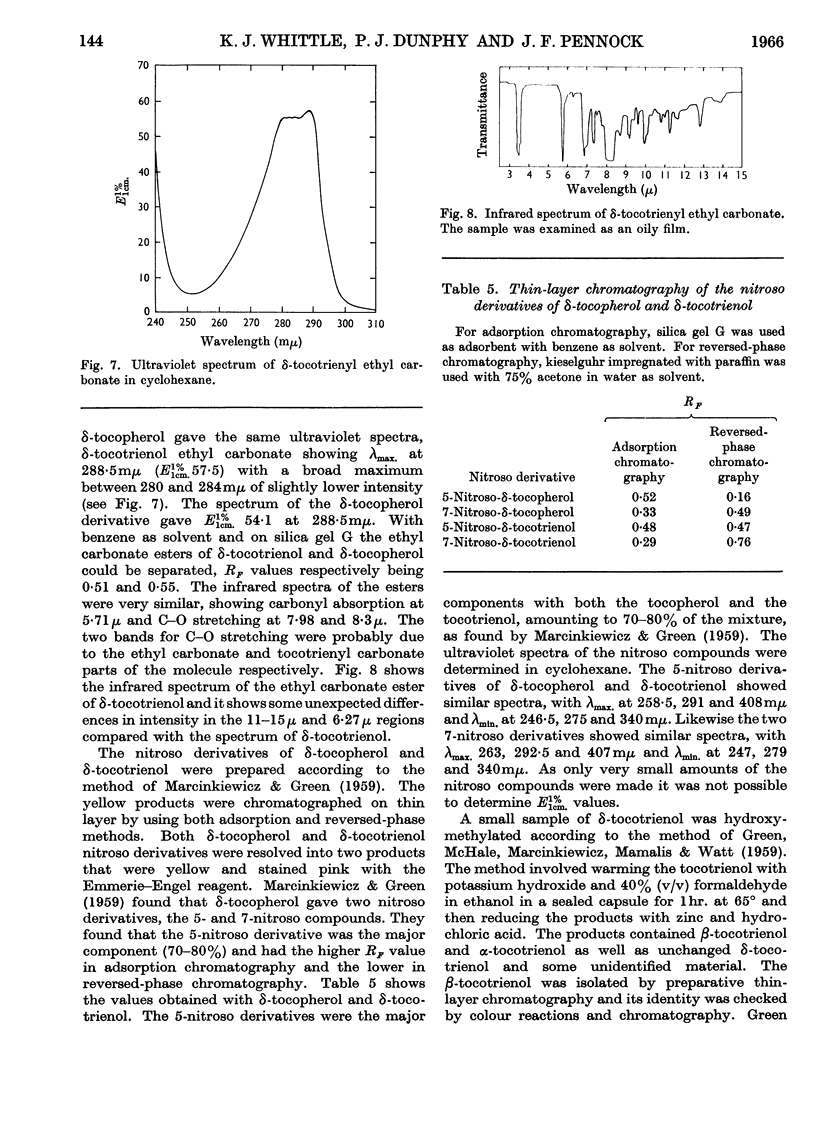
1. δ-Tocotrienol (8-methyltocotrienol) was isolated from the latex of Hevea brasiliensis. This new member of the tocopherol family is a pale-yellow oil at room temperature. 2. The properties of δ-tocotrienol are very similar to those of δ-tocopherol and the small differences can be explained by the change in side chain. 3. The ultraviolet and infrared spectra of δ-tocotrienol were determined and a conversion factor for use with the Emmerie–Engel reaction was worked out. Details are given for the chromatography of δ-tocotrienol on thin layers (adsorption and partition) and reversed-phase paper, and the nitroso derivatives were formed. 4. An ethyl carbonate ester of δ-tocotrienol was prepared and compared with a similar ester of δ-tocopherol. 5. Hydroxymethylation of δ-tocotrienol followed by reduction gave β-tocotrienol as a major product.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTH V. H. A method for separating lipid components of leaves. Biochem J. 1962 Aug;84:444–448. doi: 10.1042/bj0840444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTH V. H., BRADFORD M. P. TOCOPHEROL CONTENTS OF VEGETABLES AND FRUITS. Br J Nutr. 1963;17:575–581. doi: 10.1079/bjn19630060. [DOI] [PubMed] [Google Scholar]
- BUNYAN J., McHALE D., GREEN J., MARCINKIEWICZ S. Biological potencies of epsilon- and zeta-1-tocopherol and 5-methyltocol. Br J Nutr. 1961;15:253–257. doi: 10.1079/bjn19610030. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- GREEN J., MARCINKIEWICZ S. Eta-Tocopherol (7-methyltocol) a new tocopherol in rice. Nature. 1956 Jan 14;177(4498):86–87. doi: 10.1038/177086b0. [DOI] [PubMed] [Google Scholar]
- HERTING D. C., DRURY E. J. VITAMIN E CONTENT OF VEGETABLE OILS AND FATS. J Nutr. 1963 Dec;81:335–342. doi: 10.1093/jn/81.4.335. [DOI] [PubMed] [Google Scholar]
- IDLER D. R., BAUMANN C. A. Skin sterols. II. Isolation of delta 7-cholesterol. J Biol Chem. 1952 Apr;195(2):623–628. [PubMed] [Google Scholar]
- LAIDMAN D. L., MORTON R. A., PATERSON J. Y., PENNOCK J. F. Substance SC (ubichromenol): a naturally-occurring cyclic isomeride of ubiquinone-50. Biochem J. 1960 Mar;74:541–549. doi: 10.1042/bj0740541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock J. F., Hemming F. W., Kerr J. D. A reassessment of tocopherol in chemistry. Biochem Biophys Res Commun. 1964 Nov 30;17(5):542–548. doi: 10.1016/0006-291x(64)90062-2. [DOI] [PubMed] [Google Scholar]


