Abstract
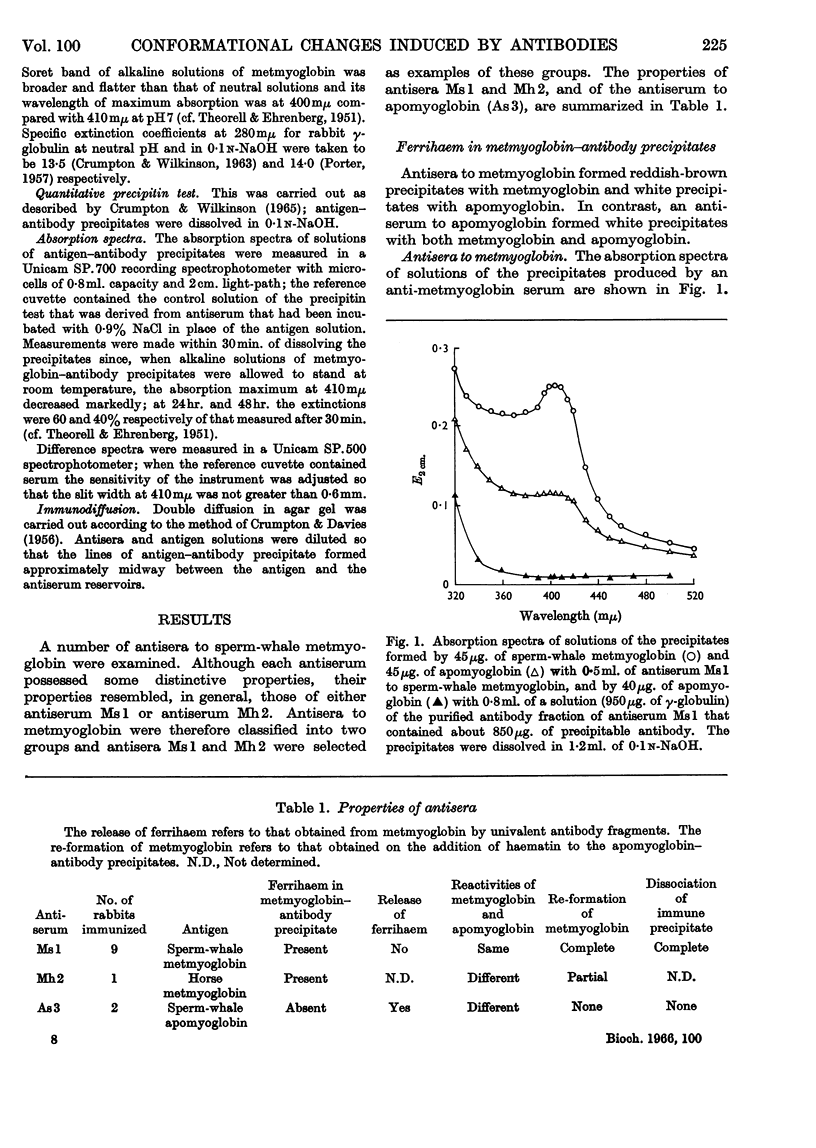
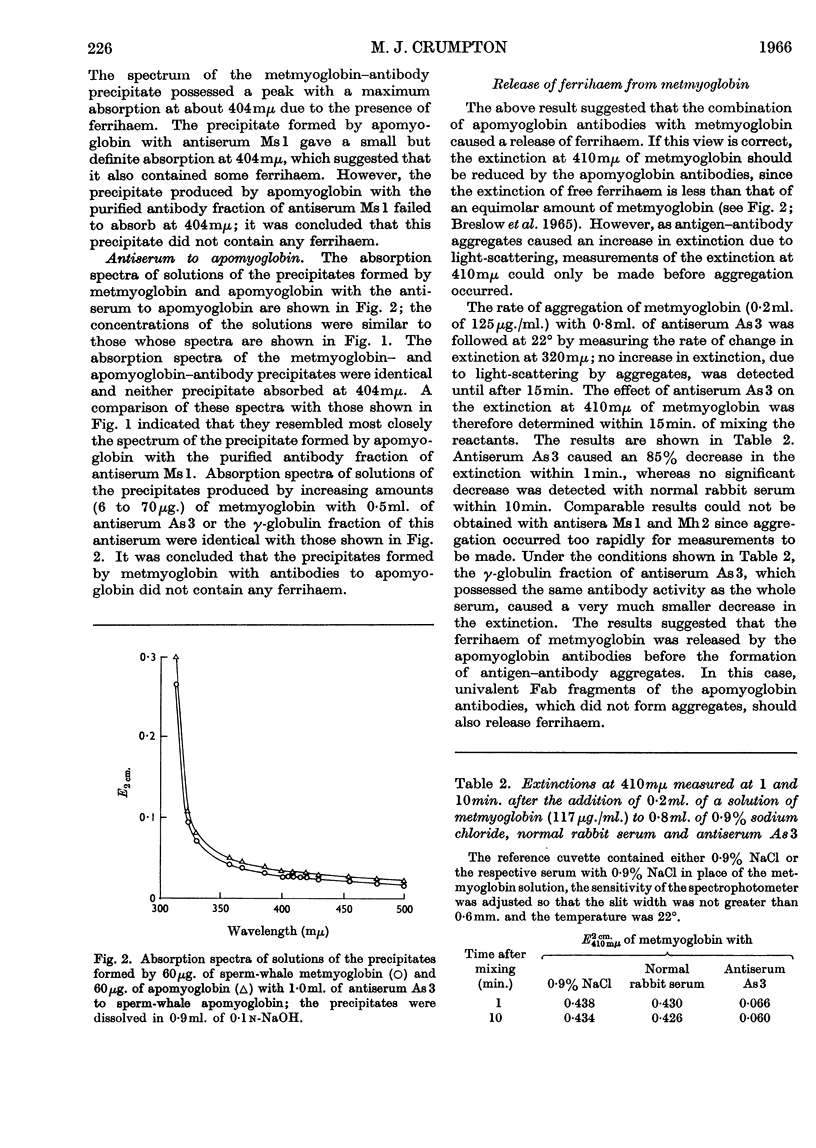
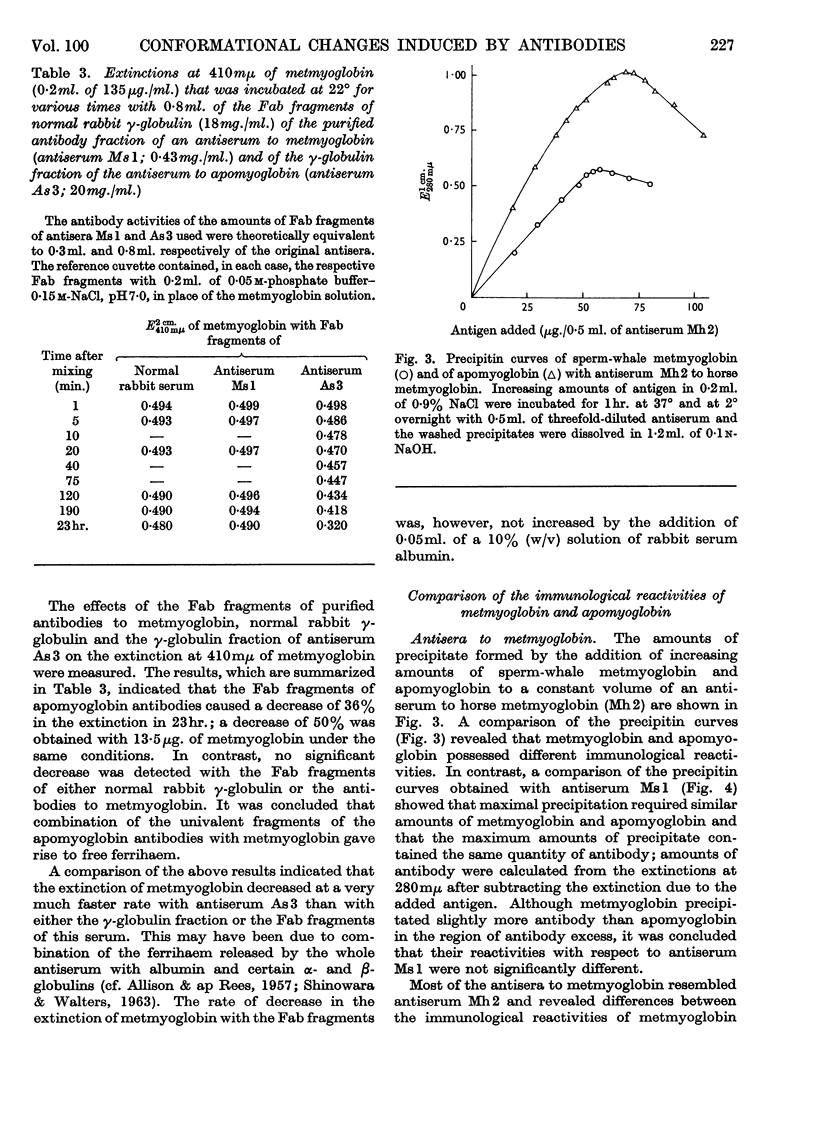
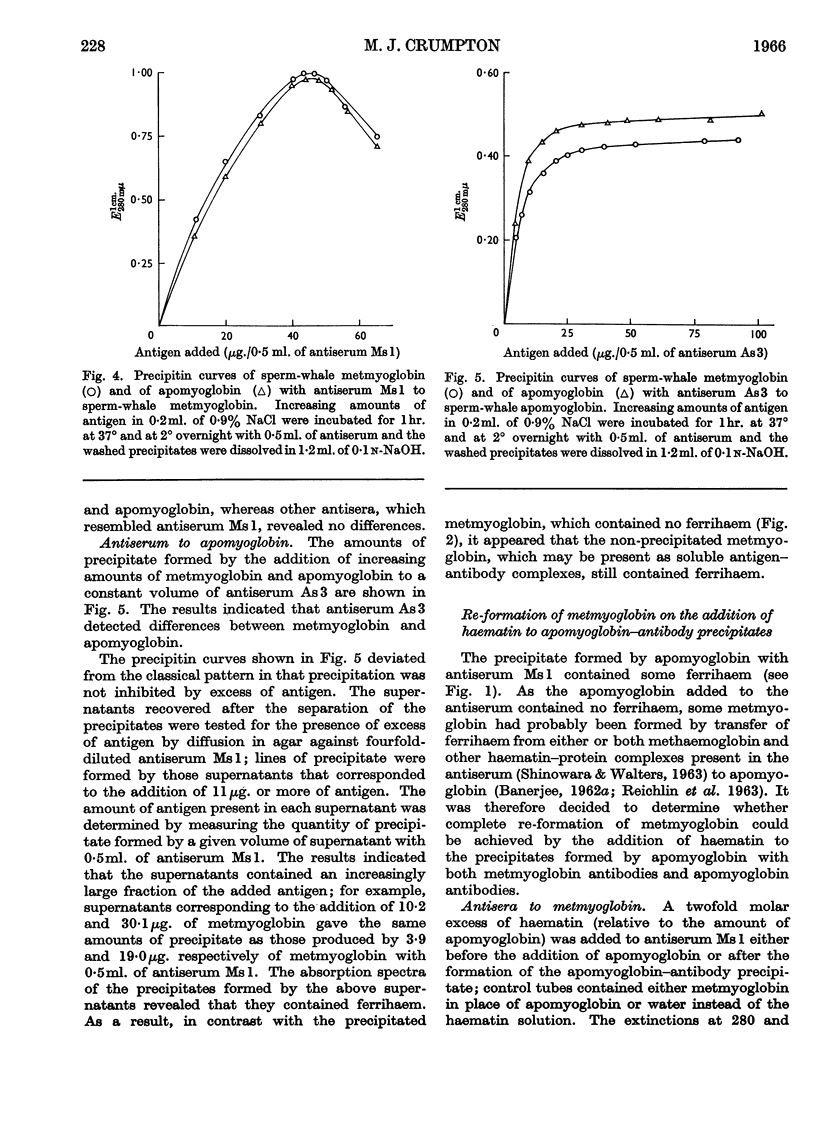
1. No ferrihaem was detected in the precipitate formed by metmyoglobin with an antiserum to apomyoglobin and the extinction at 410mμ of metmyoglobin, due to ferrihaem, was decreased by the univalent fragments of apomyoglobin antibodies. It was concluded that the combination of apomyoglobin antibodies with metmyoglobin caused the release of ferrihaem. As the removal of ferrihaem from metmyoglobin is accompanied by a conformational change, it was concluded that the conformation of metmyoglobin was altered by the apomyoglobin antibodies. 2. Antisera to metmyoglobin were divided into two groups; antisera of the first group revealed differences between the immunological reactivities of metmyoglobin and apomyoglobin, whereas no differences were detected with antisera of the second group. 3. Metmyoglobin was only partially re-formed by adding haematin to the precipitate produced by apomyoglobin with an antiserum of the first group, whereas complete re-formation of metmyoglobin was achieved in the presence of antisera of the second group. No metmyoglobin was formed on the addition of haematin to the precipitates produced by either metmyoglobin or apomyoglobin with the anti-apomyoglobin serum. 4. Immune precipitates formed by antisera to metmyoglobin dissociated at pH1·8, whereas those formed by the anti-apomyoglobin serum did not dissociate. 5. These results suggest that apomyoglobin possessed different conformations when combined with metmyoglobin antibodies and apomyoglobin antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., REES W. A. The binding of haemoglobin by plasma proteins (haptoglobins); its bearing on the renal threshold for haemoglobin and the aetiology of haemoglobinuria. Br Med J. 1957 Nov 16;2(5054):1137–1143. doi: 10.1136/bmj.2.5054.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANERJEE R. Study of hematin-globin linkage. Determination of equilibrium constants. Biochem Biophys Res Commun. 1962 Jun 19;8:114–119. doi: 10.1016/0006-291x(62)90247-4. [DOI] [PubMed] [Google Scholar]
- BANERJEE R. [Thermodynamic study of the heme-globin association. I. Dissociation equilibrium of metmyoglobin: thermodynamic data]. Biochim Biophys Acta. 1962 Oct 22;64:368–384. doi: 10.1016/0006-3002(62)90746-1. [DOI] [PubMed] [Google Scholar]
- BENNETT J. C., HABER E. Studies on antigen conformation during antibody purification. J Biol Chem. 1963 Apr;238:1362–1366. [PubMed] [Google Scholar]
- BRESLOW E., BEYCHOK S., HARDMAN K. D., GURD F. R. RELATIVE CONFORMATIONS OF SPERM WHALE METMYOGLOBIN AND APOMYOGLOBIN IN SOLUTION. J Biol Chem. 1965 Jan;240:304–309. [PubMed] [Google Scholar]
- BRESLOW E., GURD F. R. Reactivity of sperm whale metmyoglobin towards hydrogen ions and p-nitrophenyl acetate. J Biol Chem. 1962 Feb;237:371–381. [PubMed] [Google Scholar]
- CRUMPTON M. J., DAVIES D. A. An antigenic analysis of Pasteurella pestis by diffusion of antigens and antibodies in agar. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):109–134. doi: 10.1098/rspb.1956.0021. [DOI] [PubMed] [Google Scholar]
- CRUMPTON M. J., WILKINSON J. M. AMINO ACID COMPOSITIONS OF HUMAN AND RABBIT GAMMA-GLOBULINS AND OF THE FRAGMENTS PRODUCED BY REDUCTION. Biochem J. 1963 Aug;88:228–234. doi: 10.1042/bj0880228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Wilkinson J. M. The immunological activity of some of the chymotryptic peptides of sperm-whale myoglobin. Biochem J. 1965 Mar;94(3):545–556. doi: 10.1042/bj0940545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese A., Sehon A. H. Kinetic and equilibrium studies of the reaction between anti-p-nitrophenyl antibodies and a homologous hapten. Immunochemistry. 1965 Jun;2(2):135–143. doi: 10.1016/0019-2791(65)90015-7. [DOI] [PubMed] [Google Scholar]
- GIVOL D., FUCHS S., SELA M. Isolation of antibodies to antigens of low molecular weight. Biochim Biophys Acta. 1962 Sep 10;63:222–224. doi: 10.1016/0006-3002(62)90362-1. [DOI] [PubMed] [Google Scholar]
- HARRISON S. C., BLOUT E. R. REVERSIBLE CONFORMATIONAL CHANGES OF MYOGLOBIN AND APOMYOGLOBIN. J Biol Chem. 1965 Jan;240:299–303. [PubMed] [Google Scholar]
- INADA Y., SHIBATA K. The Soret band of monomeric hematin and its changes on polymerization. Biochem Biophys Res Commun. 1962 Oct 31;9:323–327. doi: 10.1016/0006-291x(62)90048-7. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE L. Determinants of specificity of proteins, nucleic acids, and polypeptides. Fed Proc. 1962 Jul-Aug;21:711–720. [PubMed] [Google Scholar]
- MICHAELIDES M. C., SHERMAN R., HELMREICH E. THE INTERACTION OF MUSCLE PHOSPHORYLASE WITH SOLUBLE ANTIBODY FRAGMENTS. J Biol Chem. 1964 Dec;239:4171–4181. [PubMed] [Google Scholar]
- NAJJAR V. A., FISHER J. The mechanism of antibody-antigen reaction. Biochim Biophys Acta. 1956 Apr;20(1):158–169. doi: 10.1016/0006-3002(56)90274-8. [DOI] [PubMed] [Google Scholar]
- NAJJAR V. A. Some aspects of antibody-antigen reactions and theoretical considerations of the immunologic response. Physiol Rev. 1963 Apr;43:243–262. doi: 10.1152/physrev.1963.43.2.243. [DOI] [PubMed] [Google Scholar]
- PERUTZ M. F., BOLTON W., DIAMOND R., MUIRHEAD H., WATSON H. C. STRUCTURE OF HAEMOGLOBIN. AN X-RAY EXAMINATION OF REDUCED HORSE HAEMOGLOBIN. Nature. 1964 Aug 15;203:687–690. doi: 10.1038/203687a0. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The isolation and properties of a fragment of bovine-serum albumin which retains the ability to combine with rabbit antiserum. Biochem J. 1957 Aug;66(4):677–686. doi: 10.1042/bj0660677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICHLIN M., BUCCI E., ANTONINI E., WYMAN J., ROSSI-FANELLI A. THE IMMUNOCHEMICAL DIFFERENCE BETWEEN HORSE OXY- AND DEOXYHAEMOGLOBIN. J Mol Biol. 1964 Sep;9:785–788. doi: 10.1016/s0022-2836(64)80184-4. [DOI] [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. IMMUNOCHEMICAL STUDIES OF HEMOGLOBIN AND MYOGLOBIN AND THEIR GLOBIN MOIETIES. Biochemistry. 1963 Sep-Oct;2:971–979. doi: 10.1021/bi00905a013. [DOI] [PubMed] [Google Scholar]
- SHINOWARA G. Y., WALTERS M. I. HEMATIN--STUDIES ON PROTEIN COMPLEXES AND DETERMINATION IN HUMAN PLASMA. Am J Clin Pathol. 1963 Aug;40:113–122. doi: 10.1093/ajcp/40.2.113. [DOI] [PubMed] [Google Scholar]


