Abstract
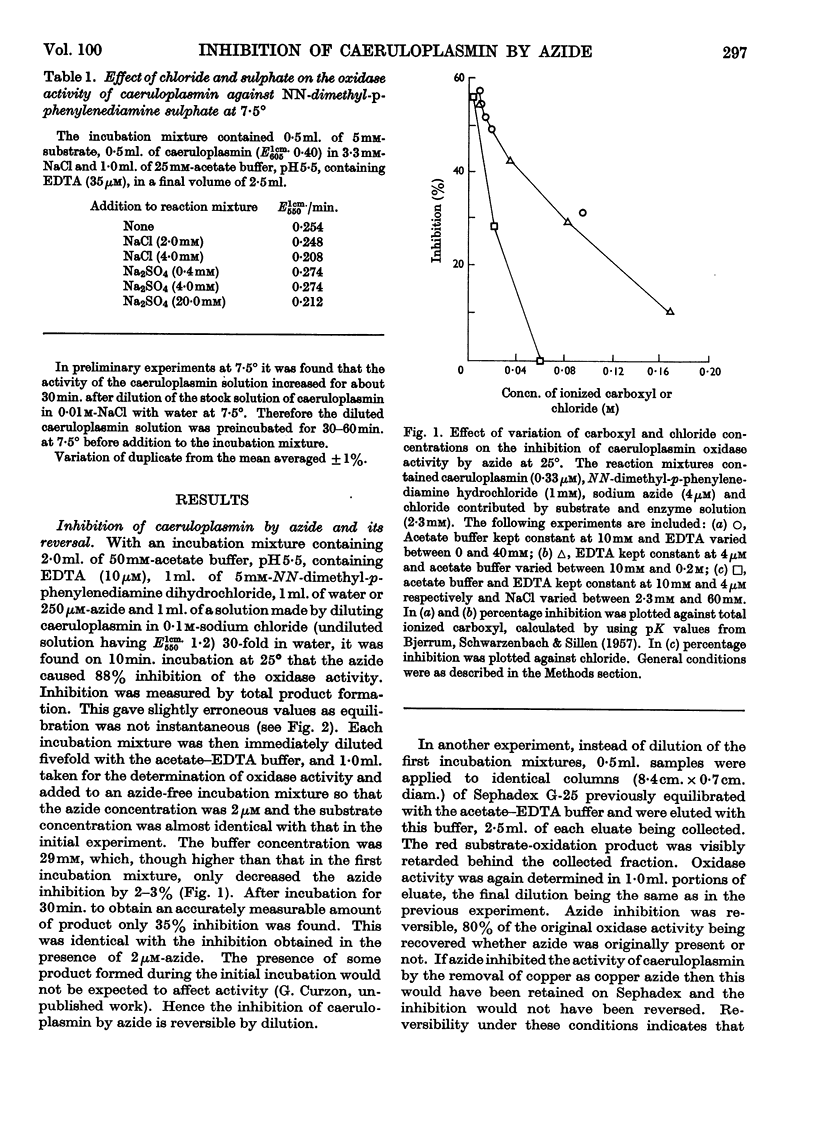
1. The inhibition of the oxidase activity of caeruloplasmin by azide was investigated at 25° and 7·5°. 2. The inhibition is reversible on dilution or Sephadex treatment, indicating a caeruloplasmin–azide complex. 3. The enzyme is protected against azide inhibition by chloride, acetate or EDTA, the last-named acting not by chelation but by a non-specific effect similar to that of acetate. 4. Lineweaver–Burk plots with different concentrations of azide are parallel. This may occur either when the enzyme–substrate complex or when a subsequent intermediate structure of the enzyme forms the inhibited complex. 5. At 7·5° inhibition may be shown not to occur until after the initial reaction of enzyme with substrate. 6. At 7·5°, the inhibition is of the mutual-depletion type, inhibitory concentrations of azide being comparable with the concentration of caeruloplasmin. It is shown that the binding of a single azide group completely inhibits a caeruloplasmin molecule. 7. An arrangement of the four valence-changing copper atoms of caeruloplasmin is proposed in which they are so close together in the cuprous form that reoxidation may occur by the simultaneous transfer of four electrons from the copper atoms to a single oxygen molecule.
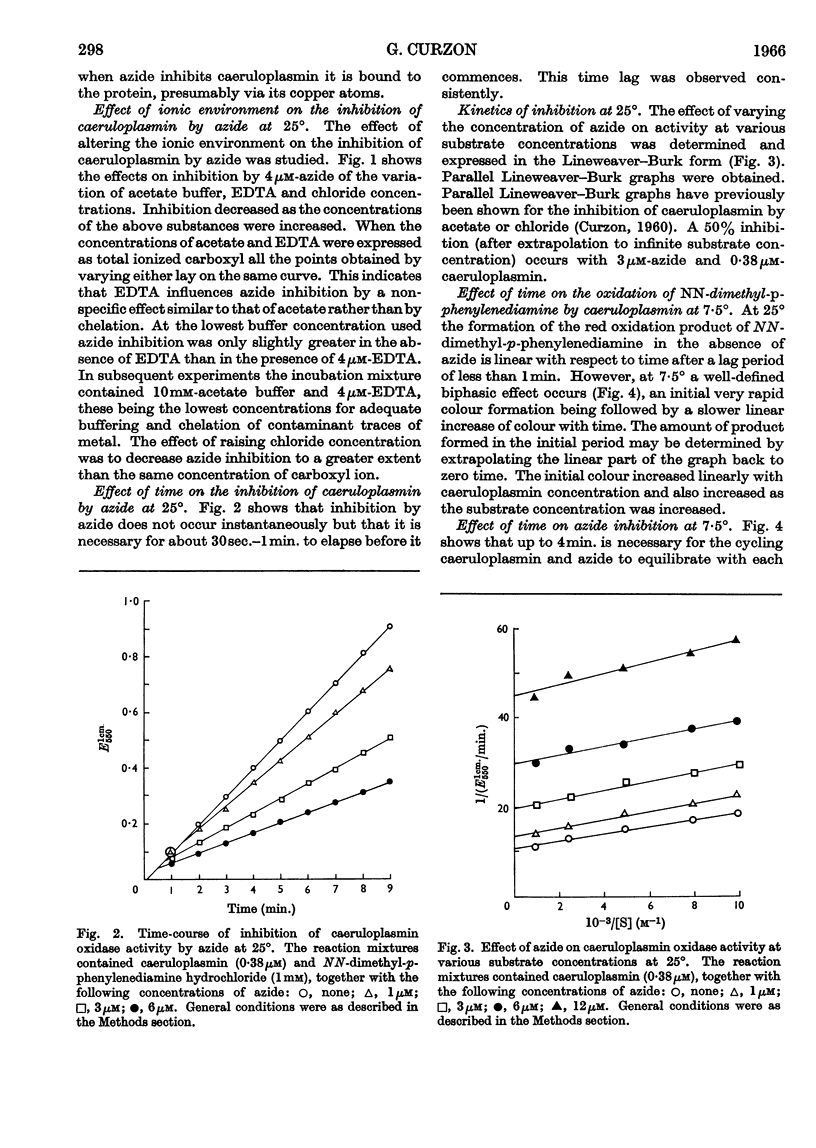
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
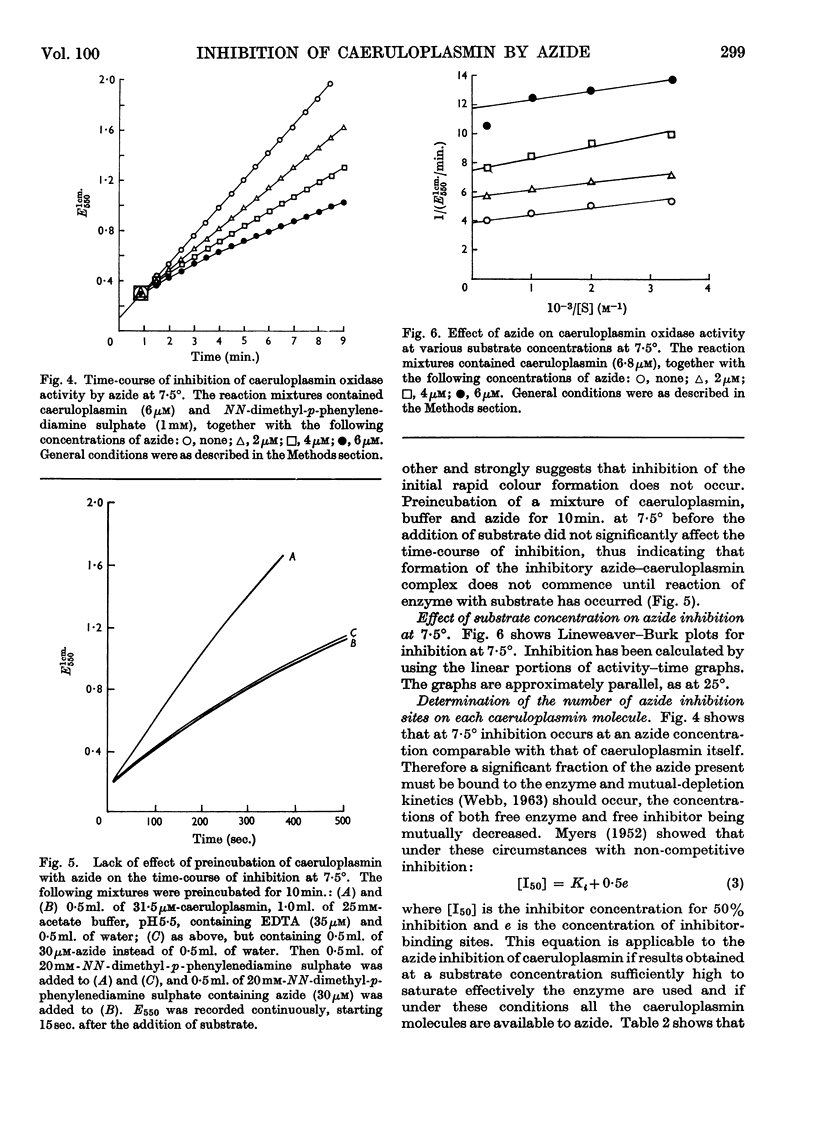
- BLUMBERG W. E., EISINGER J., AISEN P., MORELL A. G., SCHEINBERG I. H. Physical and chemical studies on ceruloplasmin. I. The relation between blue color and the valence states of copper. J Biol Chem. 1963 May;238:1675–1682. [PubMed] [Google Scholar]
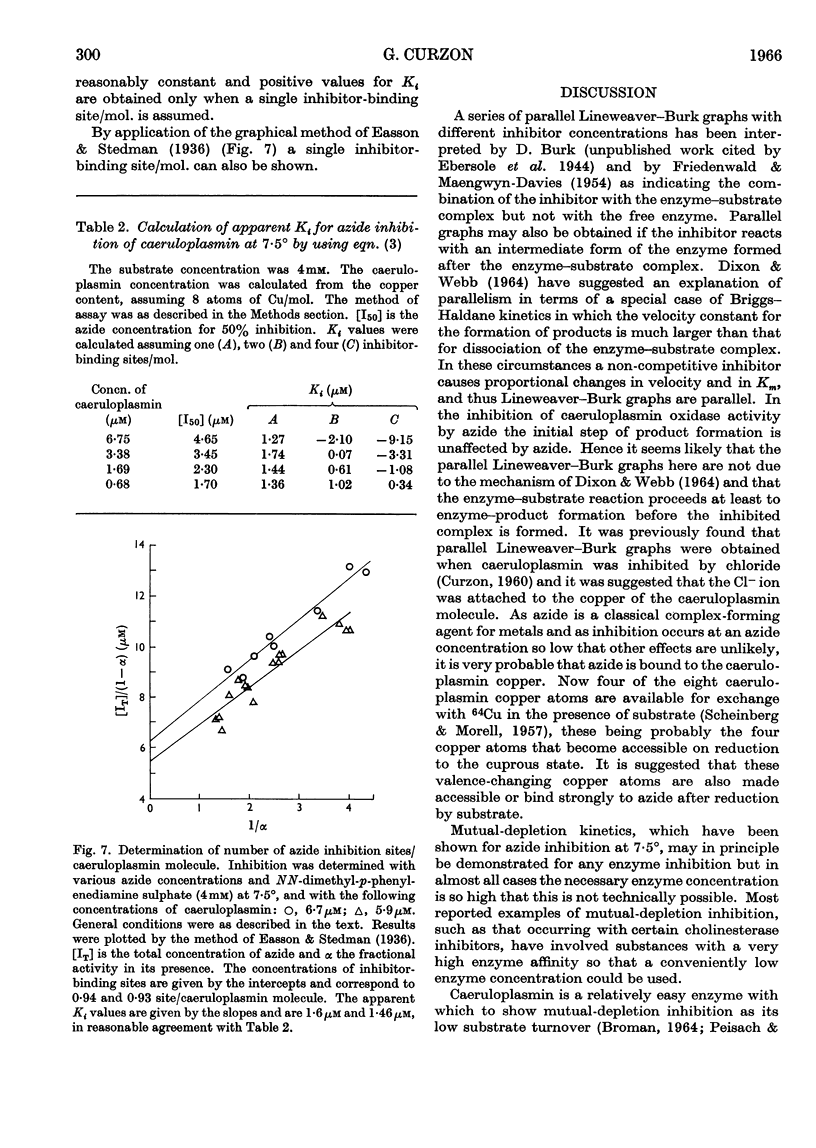
- BROMAN L., MALMSTROEM B. G., AASA R. THE ROLE OF COPPER IN THE CATALYTIC ACTION OF LACCASE AND CERULOPLASMIN. Biochim Biophys Acta. 1963 Nov 29;75:365–376. doi: 10.1016/0006-3002(63)90624-3. [DOI] [PubMed] [Google Scholar]
- BROMAN L., MALMSTROM B. G., AASA R., VANNGARD T. Quantitative electron spin resonance studies on native and denatured ceruloplasmin and laccase. J Mol Biol. 1962 Sep;5:301–310. doi: 10.1016/s0022-2836(62)80074-6. [DOI] [PubMed] [Google Scholar]
- CURZON G. Some properties of coupled iron-caeruloplasmin oxidation systems. Biochem J. 1961 Jun;79:656–663. doi: 10.1042/bj0790656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURZON G. The chemistry and biochemistry of caeruloplasmin. Proc R Soc Med. 1959 Jan;52(1):64–67. [PubMed] [Google Scholar]
- CURZON G. The effects of some ions and chelating agents on the oxidase activity of caeruloplasmin. Biochem J. 1960 Oct;77:66–73. doi: 10.1042/bj0770066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURZON G., VALLET L. The purification of human caeruloplasmin. Biochem J. 1960 Feb;74:279–287. doi: 10.1042/bj0740279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G. An investigation of the decolorization of caeruloplasmin by acid. Biochem J. 1965 Oct;97(1):151–157. doi: 10.1042/bj0970151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH H. F., KASPER C. B., WALSH D. A. Rapid method for preparation of crystalline human ceruloplasmin from Cohn fraction IV-1. Arch Biochem Biophys. 1962 Oct;99:132–135. doi: 10.1016/0003-9861(62)90255-2. [DOI] [PubMed] [Google Scholar]
- Frieden E., Osaki S., Kobayashi H. Copper proteins and oxygen. Correlations between structure and function of the copper oxidases. J Gen Physiol. 1965 Sep;49(1 Suppl):213–252. doi: 10.1085/jgp.49.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISEN P., MORELL A. G. PHYSICAL AND CHEMICAL STUDIES ON CERULOPLASMIN. 3. A STABILIZING COPPER-COPPER INTERACTION IN CERULOPLASMIN. J Biol Chem. 1965 May;240:1974–1978. [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- MORELL A. G., SCHEINBERG I. H. Preparation of an apoprotein from ceruloplasmin by reversible dissociation of copper. Science. 1958 Mar 14;127(3298):588–590. doi: 10.1126/science.127.3298.588-a. [DOI] [PubMed] [Google Scholar]
- MOSBACH R. Purification and some properties of laccase from Polyporus versicolor. Biochim Biophys Acta. 1963 Jun 11;73:204–212. doi: 10.1016/0006-3002(63)90304-4. [DOI] [PubMed] [Google Scholar]
- MYERS D. K. [Studies on cholinesterase. 7. Determination of the molar concentration of pseudo-cholinesterase in serum]. Biochem J. 1952 Jun;51(3):303–311. doi: 10.1042/bj0510303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEISACH J., LEVINE W. G. A COMPARISON OF THE ENZYMIC ACTIVITIES OF PIG CERULOPLASMIN AND RHUS VERNICIFERA LACCASE. J Biol Chem. 1965 Jun;240:2284–2289. [PubMed] [Google Scholar]
- SCHEINBERG I. H., MORELL A. G. Exchange of ceruloplasmin copper with ionic Cu64 with reference to Wilson's disease. J Clin Invest. 1957 Aug;36(8):1193–1201. doi: 10.1172/JCI103515. [DOI] [PMC free article] [PubMed] [Google Scholar]


