Abstract
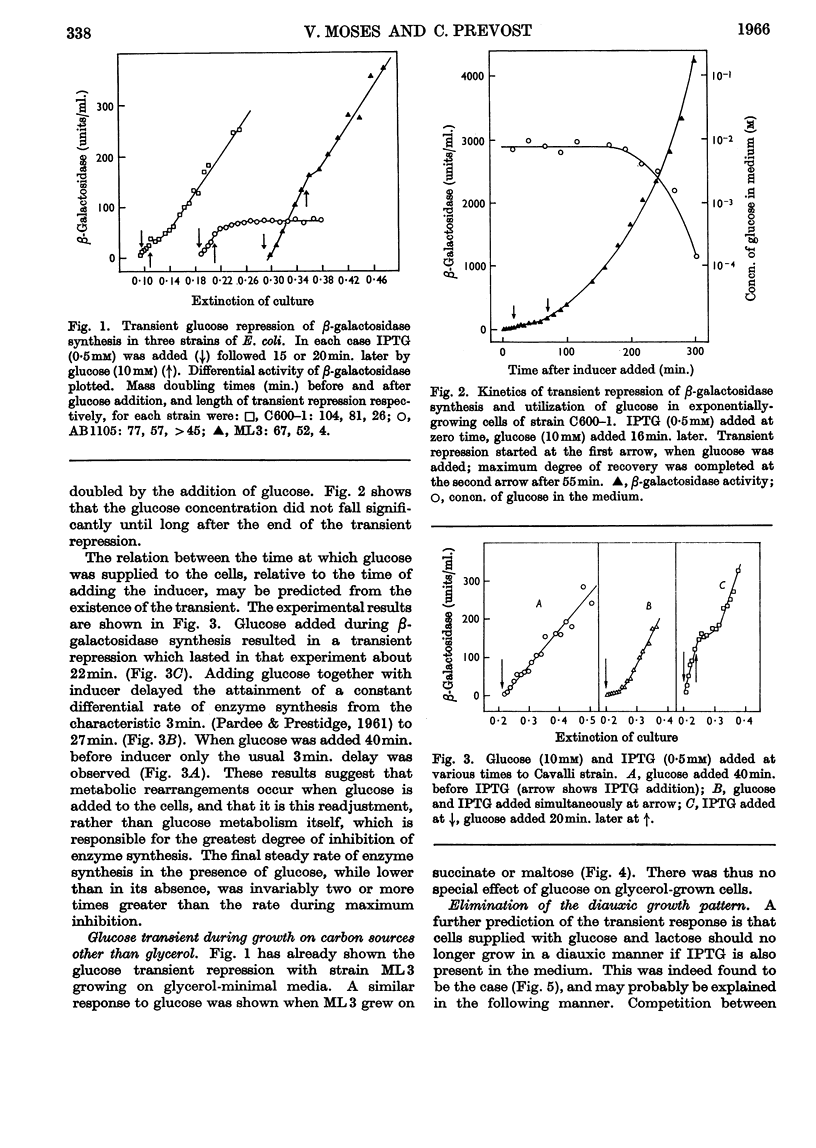
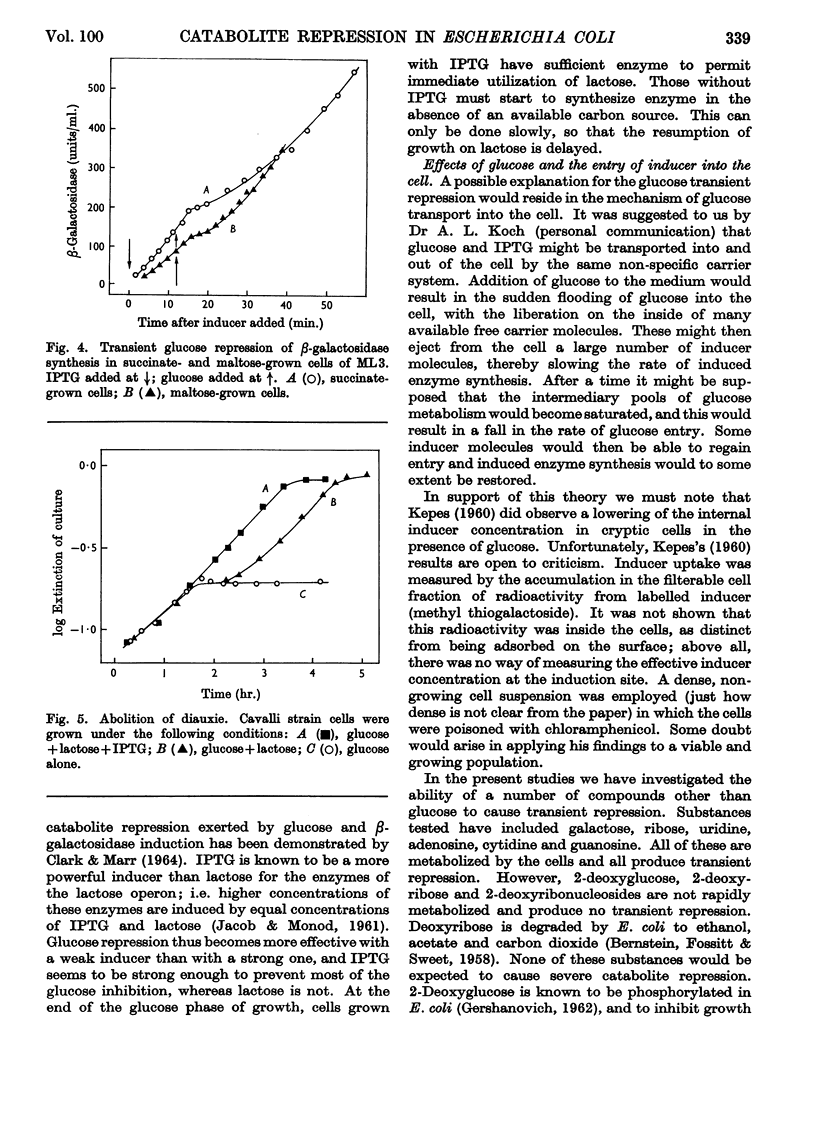
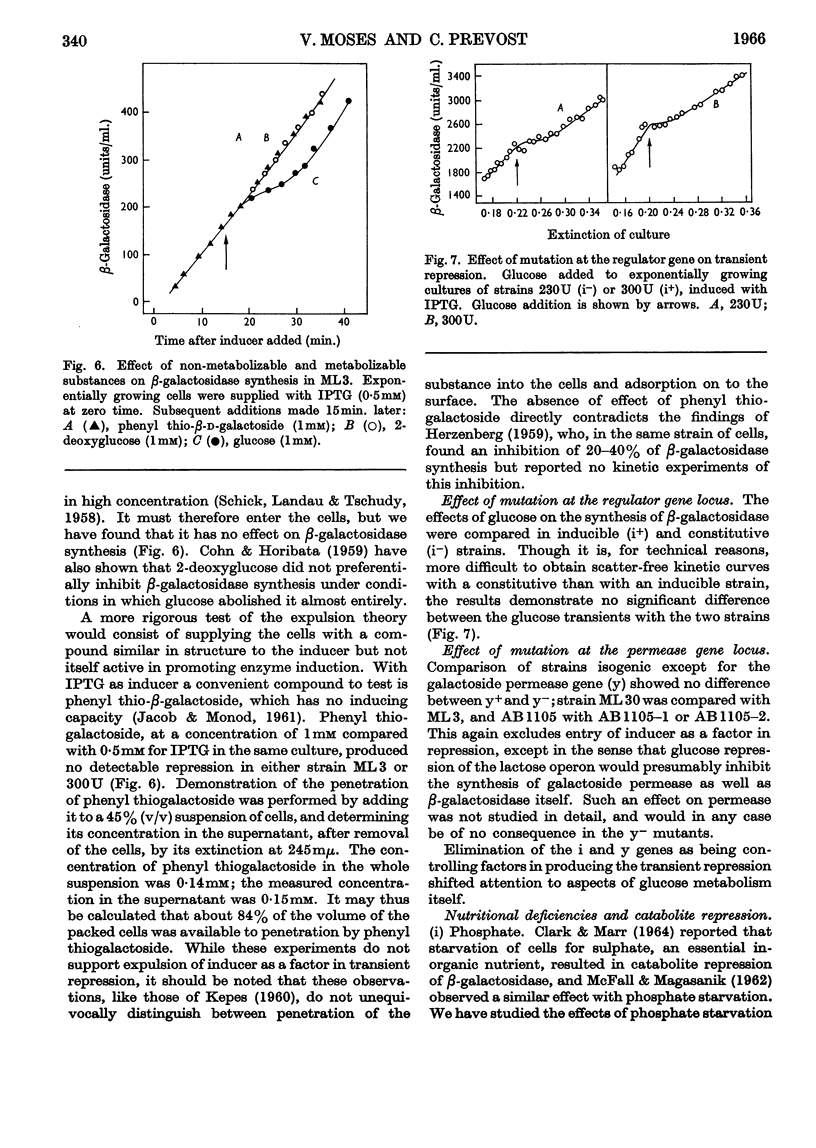
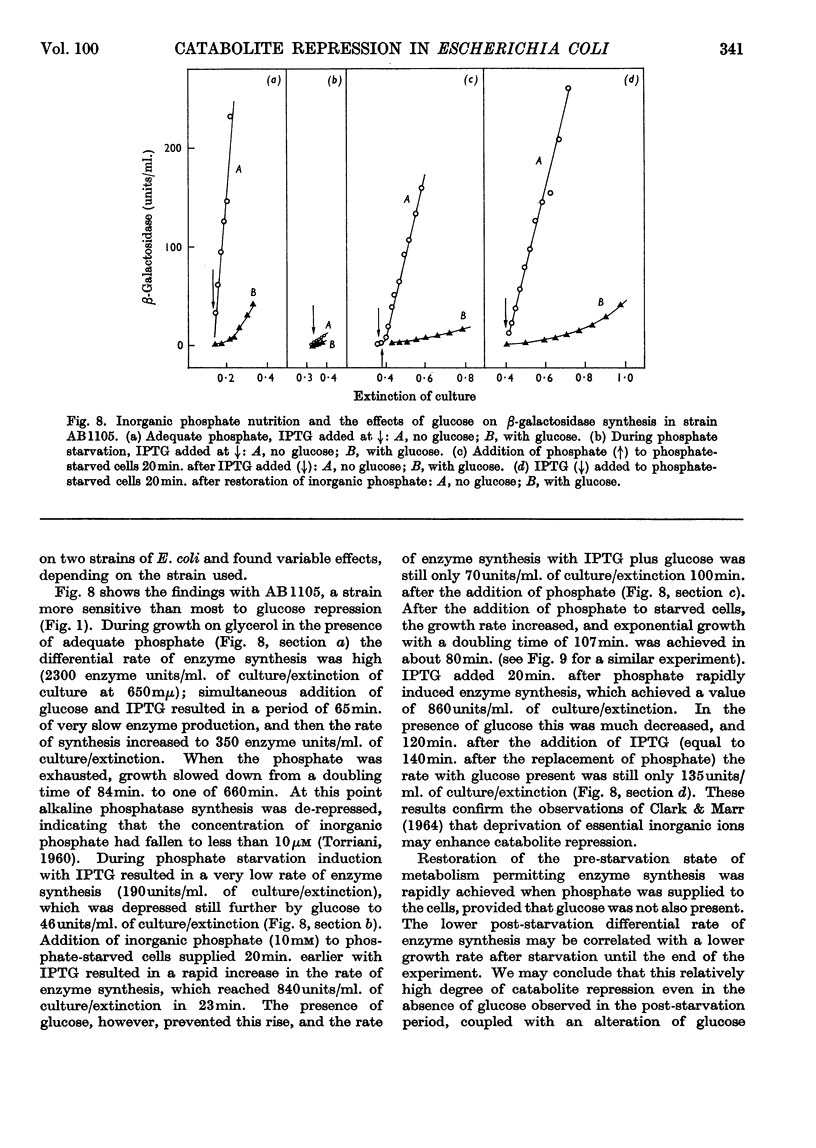
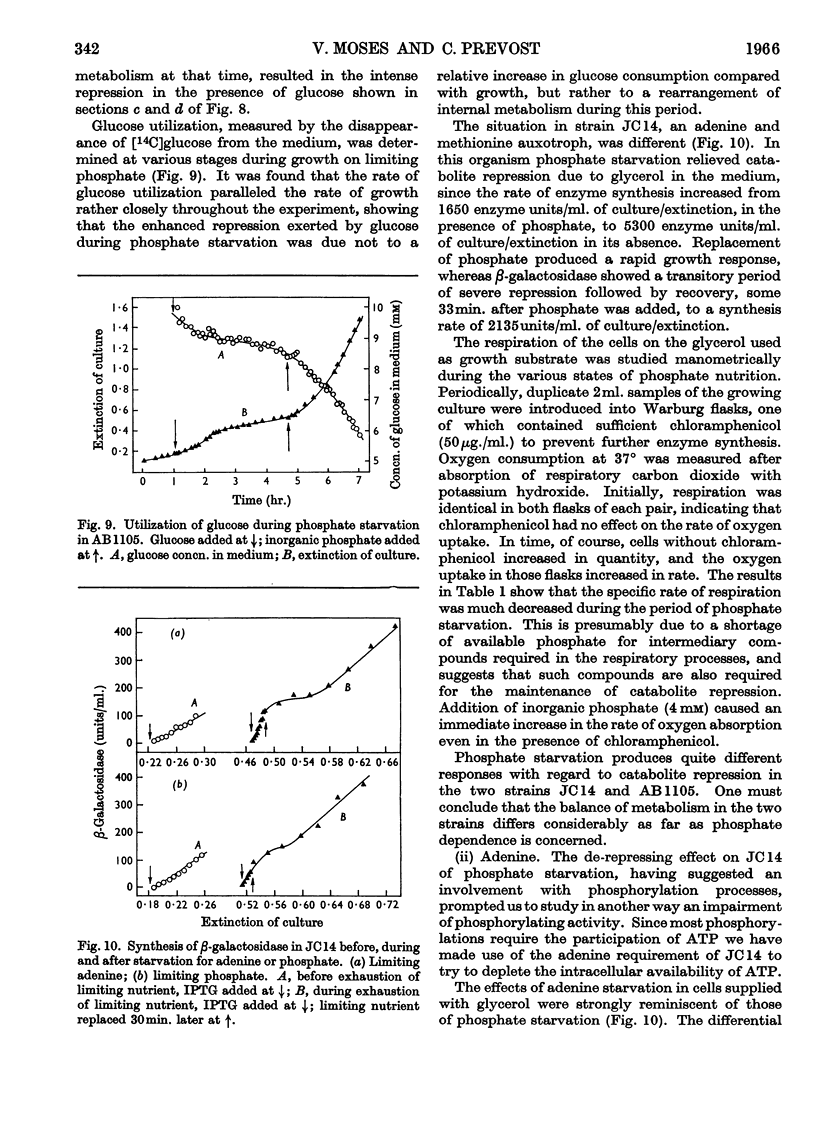
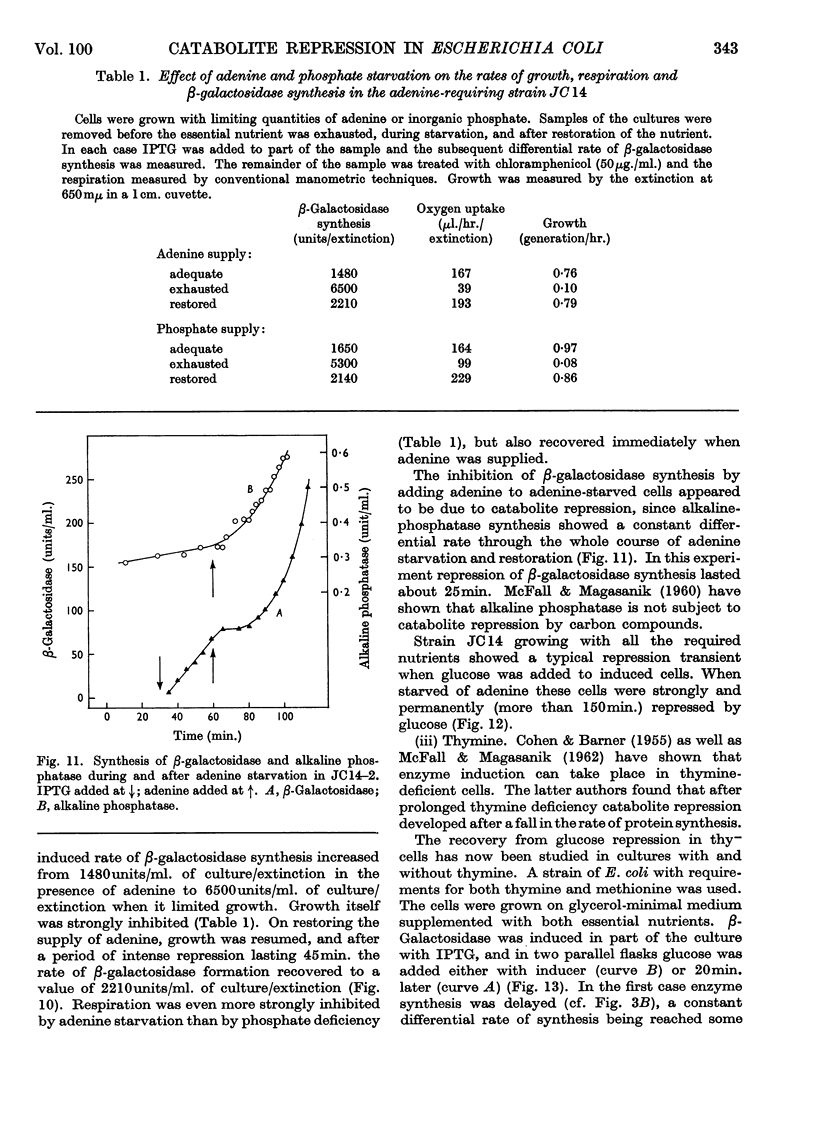
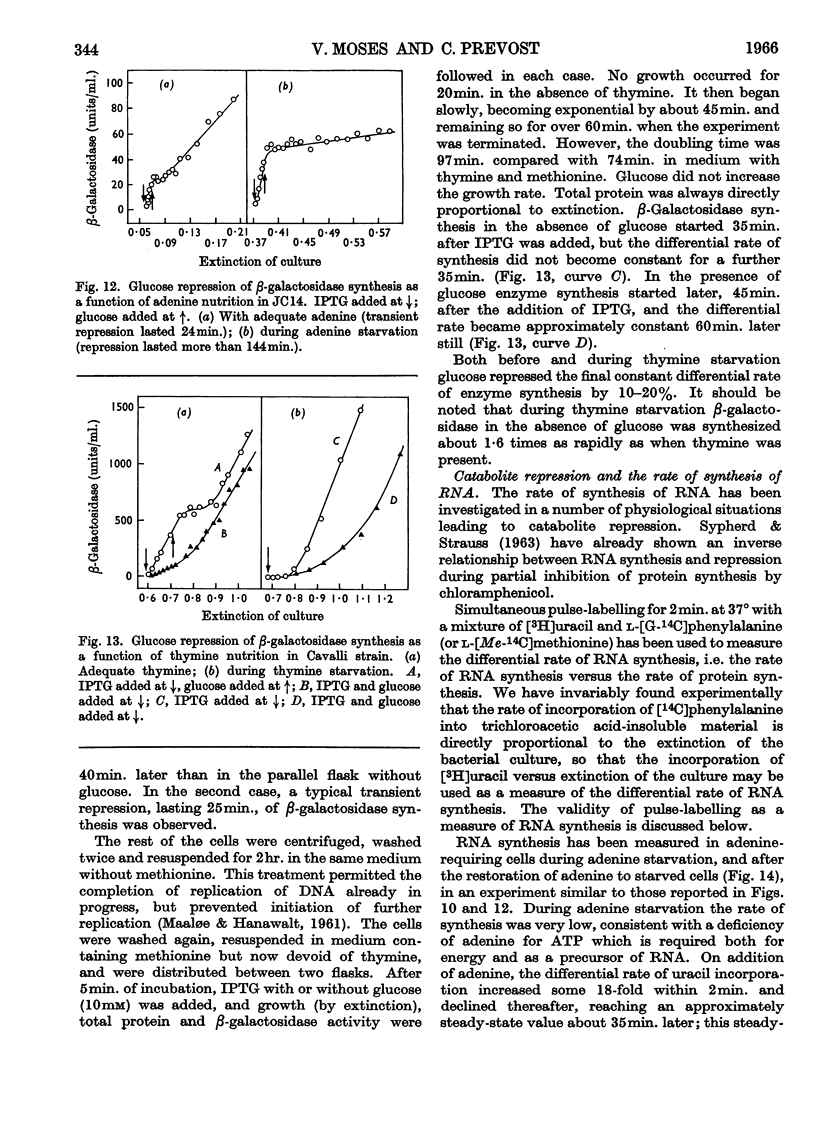
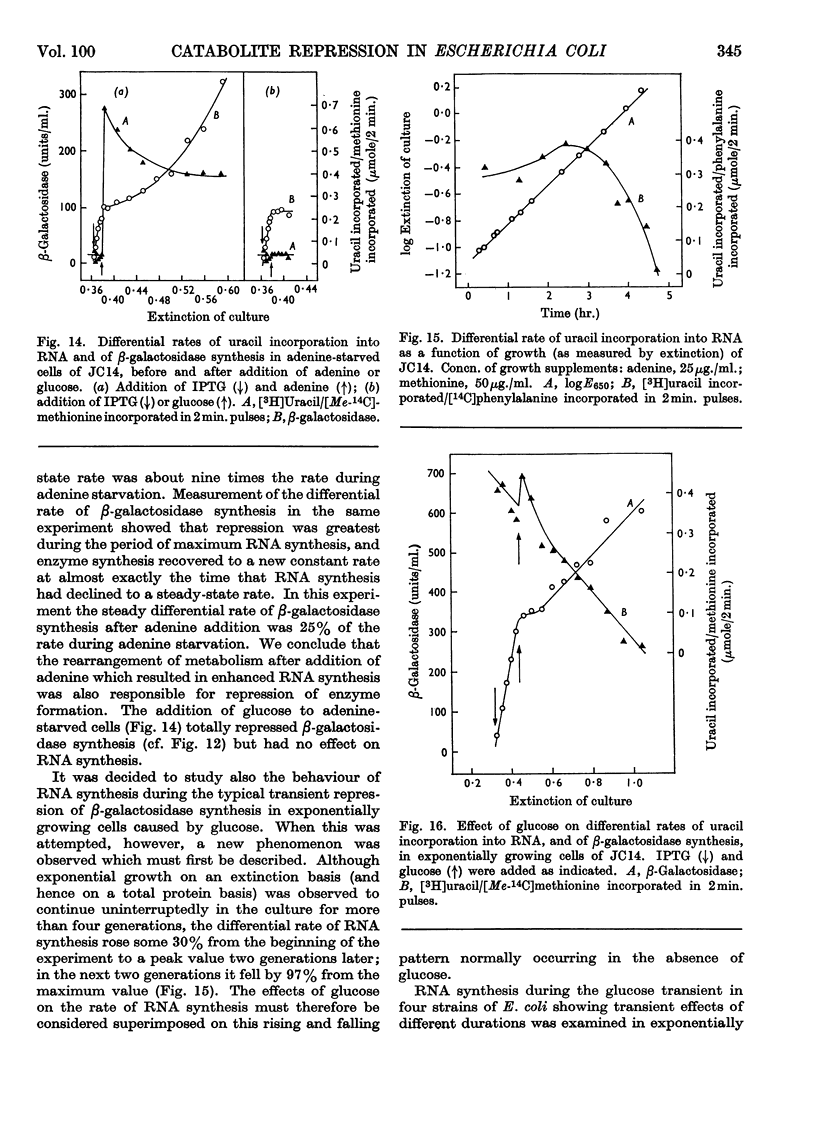
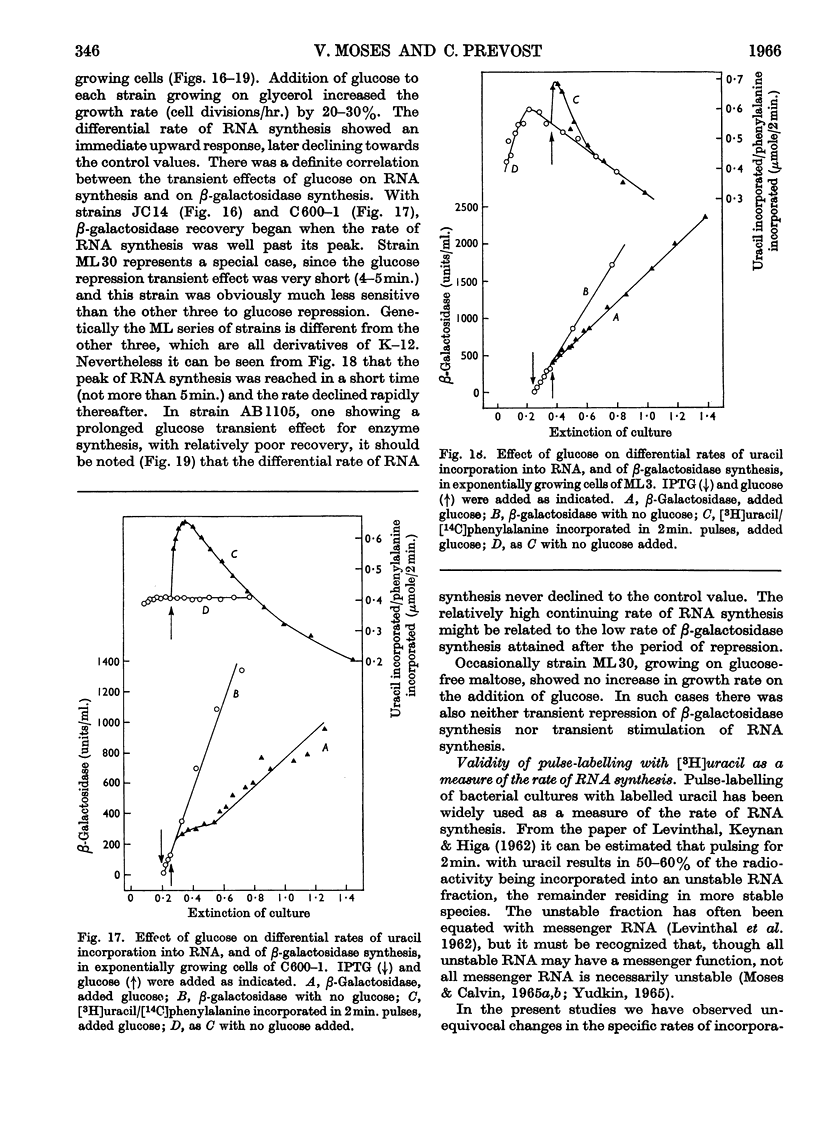
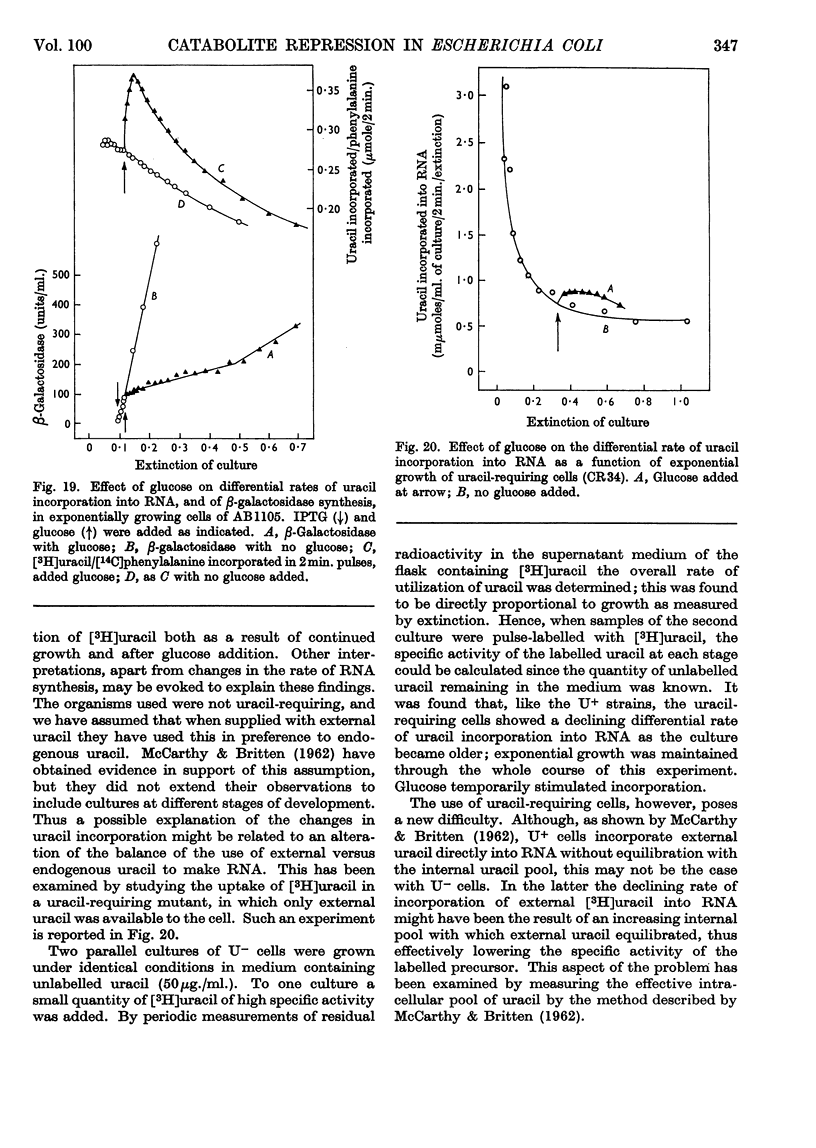
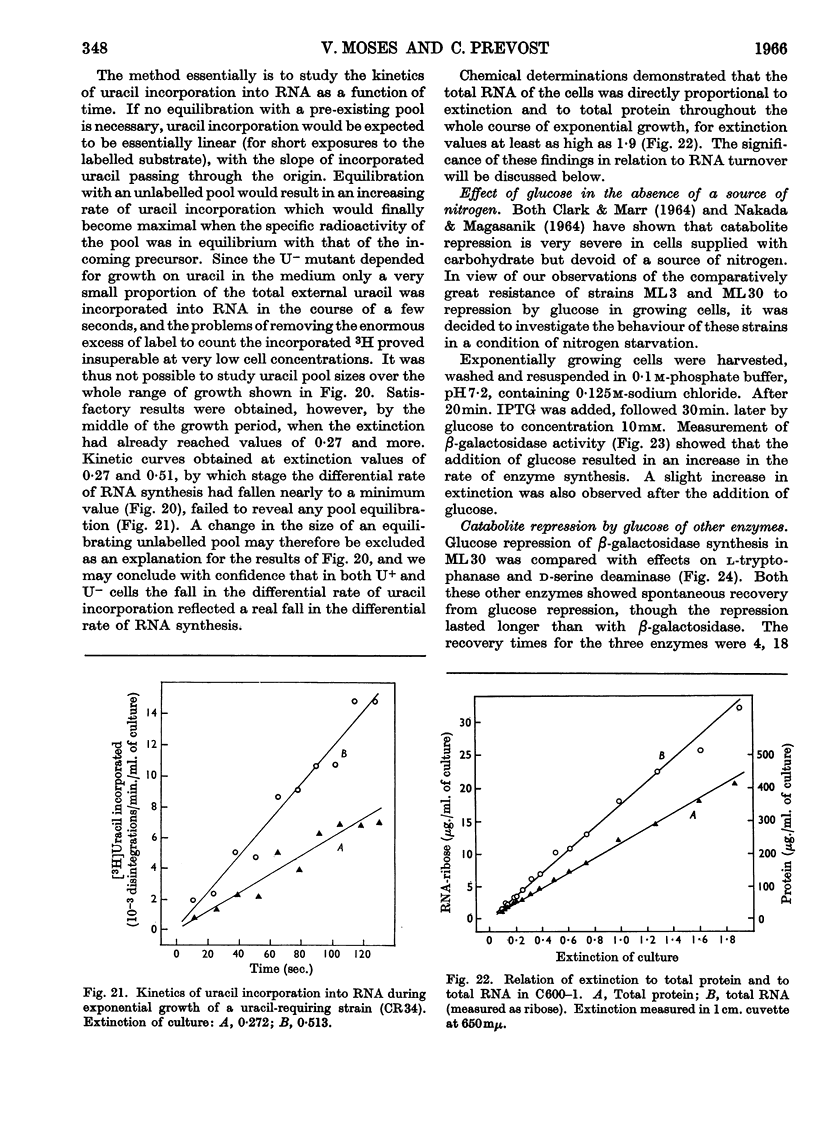
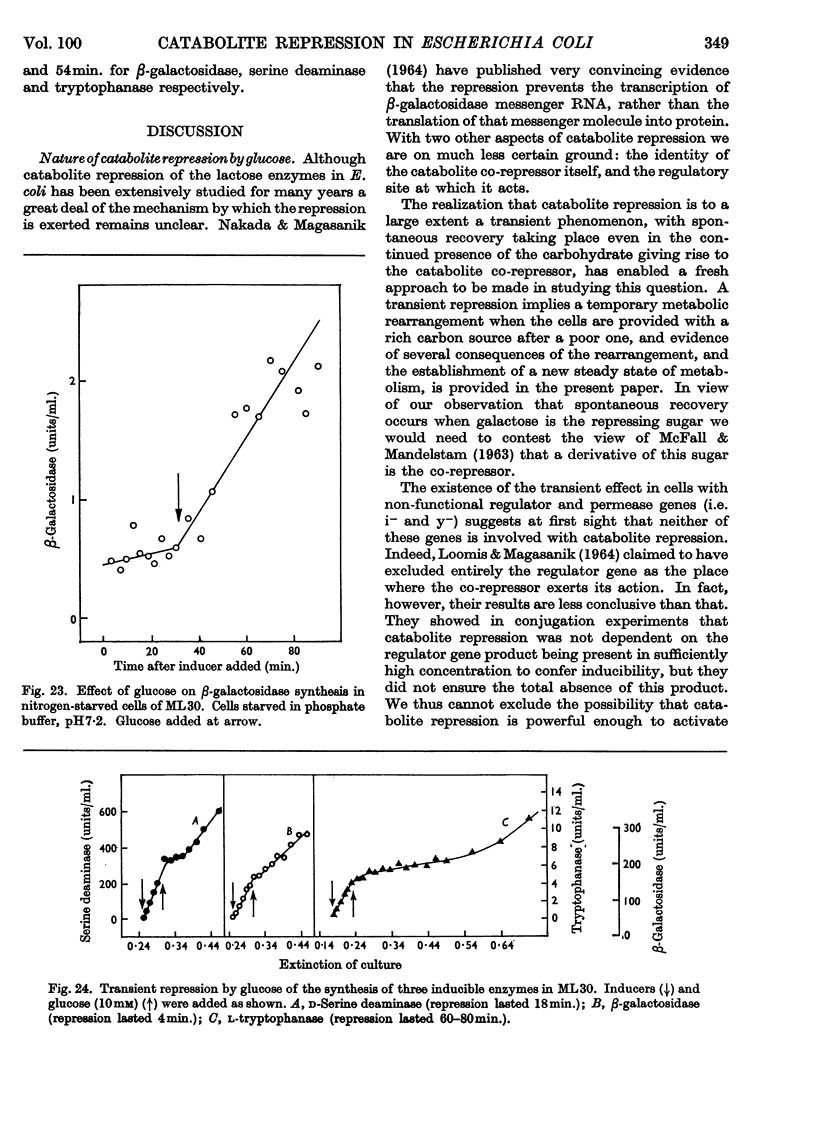
1. Repression by glucose of β-galactosidase synthesis is spontaneously reversible in all strains of Escherichia coli examined long before the glucose has all been consumed. The extent of recovery and the time necessary for reversal differ among various strains. Other inducible enzymes show similar effects. 2. This transient effect of glucose repression is observed in constitutive (i−) and permease-less (y−) cells as well as in the corresponding i+ and y+ strains. 3. Repression is exerted by several rapidly metabolizable substrates (galactose, ribose and ribonucleosides) but not by non-metabolized or poorly metabolized compounds (2-deoxyglucose, 2-deoxyribose, phenyl thio-β-galactoside and 2-deoxyribonucleosides). 4. The transient repression with glucose is observed in inducible cells supplied with a powerful inducer of β-galactosidase synthesis (e.g. isopropyl thio-β-galactoside) but not with a weak inducer (lactose); in the latter instance glucose repression is permanent. Diauxic growth on glucose plus lactose can be abolished by including isopropyl thio-β-galactoside in the medium. 5. In some strains phosphate starvation increases catabolite repression; in others it relieves it. Adenine starvation in an adenine-requiring mutant also relieves catabolite repression by glycerol but not that by glucose. Restoration of phosphate or adenine to cells starved of these nutrients causes a pronounced temporary repression. Alkaline-phosphatase synthesis is not affected by the availability of adenine. 6. During periods of transient repression of induced enzyme synthesis the differential rate of RNA synthesis, measured by labelled uracil incorporation in 2min. pulses, shows a temporary rise. 7. The differential rate of uracil incorporation into RNA falls during exponential growth of batch cultures of E. coli. This is equally true for uracil-requiring and non-requiring strains. The fall in the rate of incorporation has been shown to be due to a real fall in the rate of RNA synthesis. The significance of the changes in the rate of RNA synthesis is discussed. 8. A partial model of catabolite repression is presented with suggestions for determining the chemical identification of the catabolite co-repressor itself.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELOZERSKY A. N., SPIRIN A. S. A correlation between the compositions of deoxyribonucleic and ribonucleic acids. Nature. 1958 Jul 12;182(4628):111–112. doi: 10.1038/182111a0. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN I. A., FOSSITT D., SWEET D. Bacterial degradation of deoxyribose-C 14. J Biol Chem. 1958 Nov;233(5):1199–1202. [PubMed] [Google Scholar]
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALVIN M., MASSINI P. The path of carbon in photosynthesis. XX. The steady state. Experientia. 1952 Dec 15;8(12):445–457. doi: 10.1007/BF02139287. [DOI] [PubMed] [Google Scholar]
- CLARKE P. H., BRAMMAR W. J. REGULATION OF BACTERIAL ENZYME SYNTHESIS BY INDUCTION AND REPRESSION. Nature. 1964 Sep 12;203:1153–1155. doi: 10.1038/2031153a0. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., BARNER H. Enzymatic adaptation in a thymine requiring strain of Escherichia coli. J Bacteriol. 1955 Jan;69(1):59–66. doi: 10.1128/jb.69.1.59-66.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J. THE INFLUENCE OF NITRATE AND NITRITE REDUCTION ON CATABOLITE REPRESSION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:553–566. doi: 10.1016/0304-4165(65)90025-5. [DOI] [PubMed] [Google Scholar]
- ELSON D., TRENT L. W., CHARGAFF E. The nucleotide composition of pentose nucleic acids in different cellular fractions. Biochim Biophys Acta. 1955 Jul;17(3):362–366. doi: 10.1016/0006-3002(55)90384-x. [DOI] [PubMed] [Google Scholar]
- GERSHANOVICH V. N. [On the permeability of the bacterial cell of Escherichia coli to 2-D-deoxyglucose]. Biokhimiia. 1962 Nov-Dec;27:1023–1031. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOOMIS W. F., Jr, MAGASANIK B. THE RELATION OF CATABOLITE REPRESSION TO THE INDUCTION SYSTEM FOR BETA-GALACTOSIDASE IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:417–426. doi: 10.1016/s0022-2836(64)80205-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Genetic control of catabolite repression of the lac operon in Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 12;20(2):230–234. doi: 10.1016/0006-291x(65)90351-7. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E., MAGASANIK B. Thymine starvation and enzyme synthesis. Biochim Biophys Acta. 1960 Dec 18;45:610–612. doi: 10.1016/0006-3002(60)91505-5. [DOI] [PubMed] [Google Scholar]
- MCFALL E., MANDELSTAM J. SPECIFIC METABOLIC REPRESSION OF THREE INDUCED ENZYMES IN ESCHERICHIA COLI. Biochem J. 1963 Nov;89:391–398. doi: 10.1042/bj0890391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSES V., SMITH M. J. Uncoupling reagents and metabolism. 2. Effects of 2:4-dinitrophenol and salicylate on glucose metabolism in baker's yeast. Biochem J. 1960 Sep;76:585–594. doi: 10.1042/bj0760585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSES V. [14C] Glucose metabolism in fungal cells. J Gen Microbiol. 1959 Apr;20(2):184–196. doi: 10.1099/00221287-20-2-184. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses V., Calvin M. Lifetime of bacterial messenger ribonucleic acid. J Bacteriol. 1965 Nov;90(5):1205–1217. doi: 10.1128/jb.90.5.1205-1217.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- PINSKY M. J., STOKES J. L. The influence of age of enzymatic adaptation in microorganisms. J Bacteriol. 1952 Sep;64(3):337–345. doi: 10.1128/jb.64.3.337-345.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTMAN W. E., HASSID W. Z. Isolation and purification of radioactive sugars by means of paper chromatography. J Biol Chem. 1952 May;196(2):749–752. [PubMed] [Google Scholar]
- Rao S., Bhargava P. M. The effect of bacterial concentration on the uptake of labelled arginine and glucose by Escherichia coli. J Gen Microbiol. 1965 Aug;40(2):219–225. doi: 10.1099/00221287-40-2-219. [DOI] [PubMed] [Google Scholar]
- SCHICK M., LANDAU B., TSCHUDY D. P. Effect of hexose analogues on the growth of Escherichia coli. J Bacteriol. 1958 Apr;75(4):414–416. doi: 10.1128/jb.75.4.414-416.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYPHERD P. S., STRAUSS N. THE ROLE OF RNA IN REPRESSION OF ENZYME SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1059–1066. doi: 10.1073/pnas.50.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- TORRIANI A., ROTHMAN F. Mutants of Escherichia coli constitutive for alkaline phosphatase. J Bacteriol. 1961 May;81:835–836. doi: 10.1128/jb.81.5.835-836.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin M. D. Amino acid incorporation in Bacillus megaterium treated with actinomycin. Biochim Biophys Acta. 1965 Aug 10;103(4):705–707. doi: 10.1016/0005-2787(65)90093-6. [DOI] [PubMed] [Google Scholar]



