Abstract
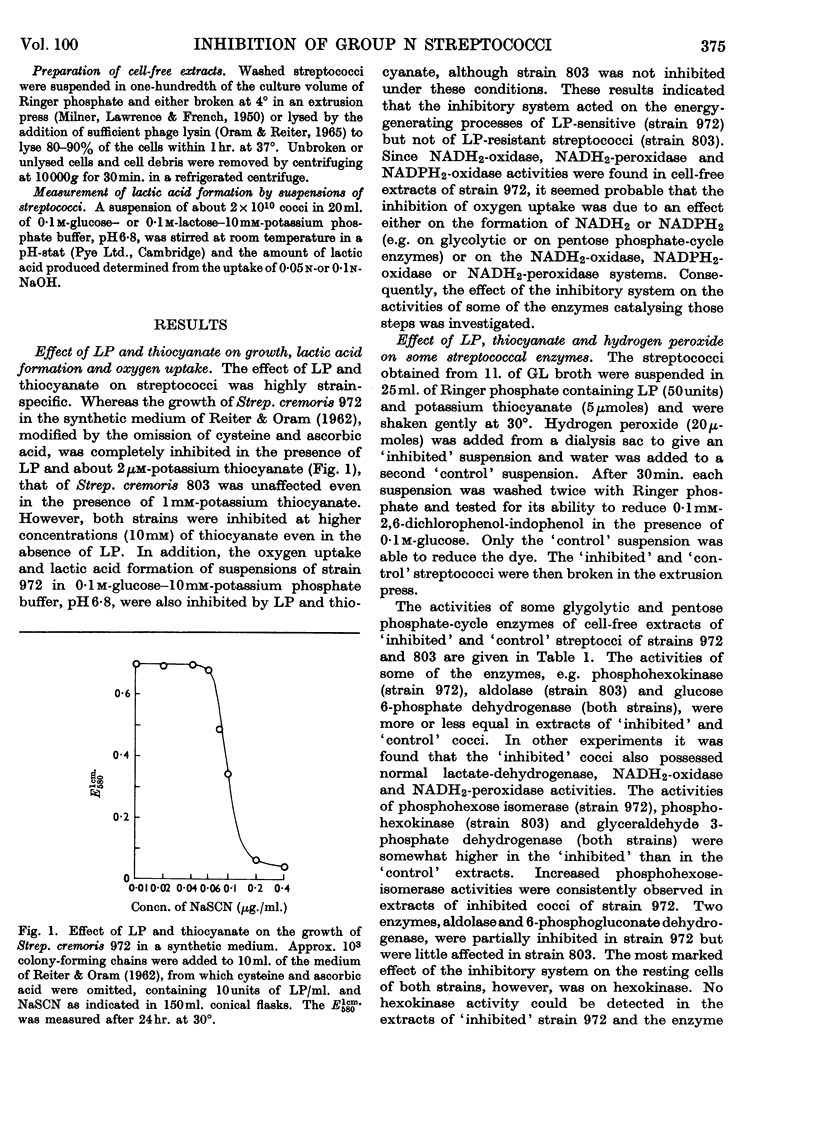
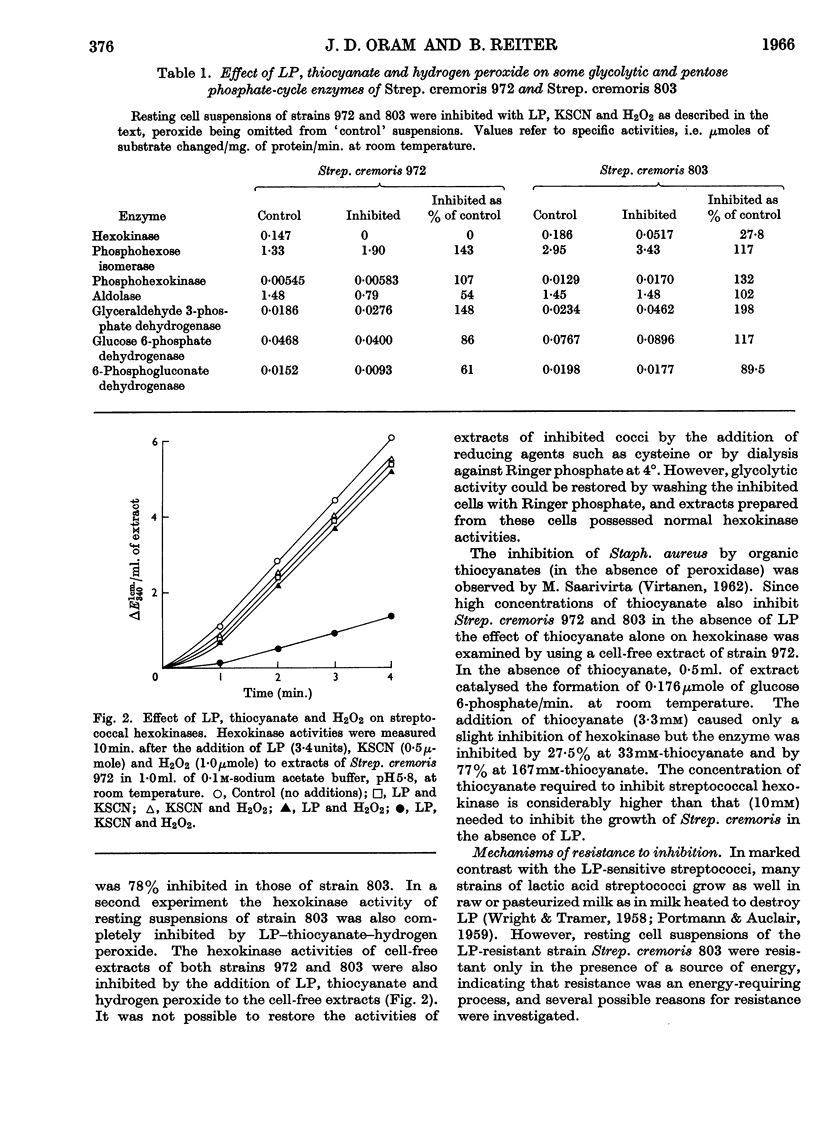
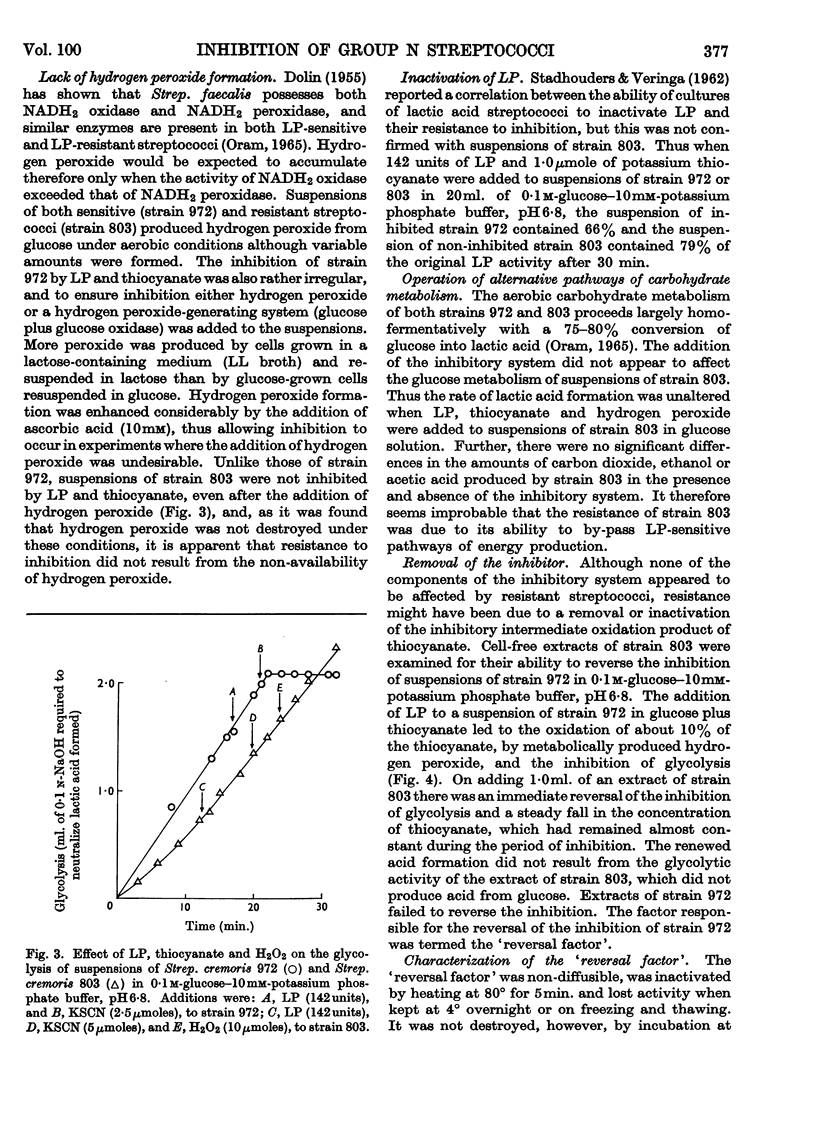
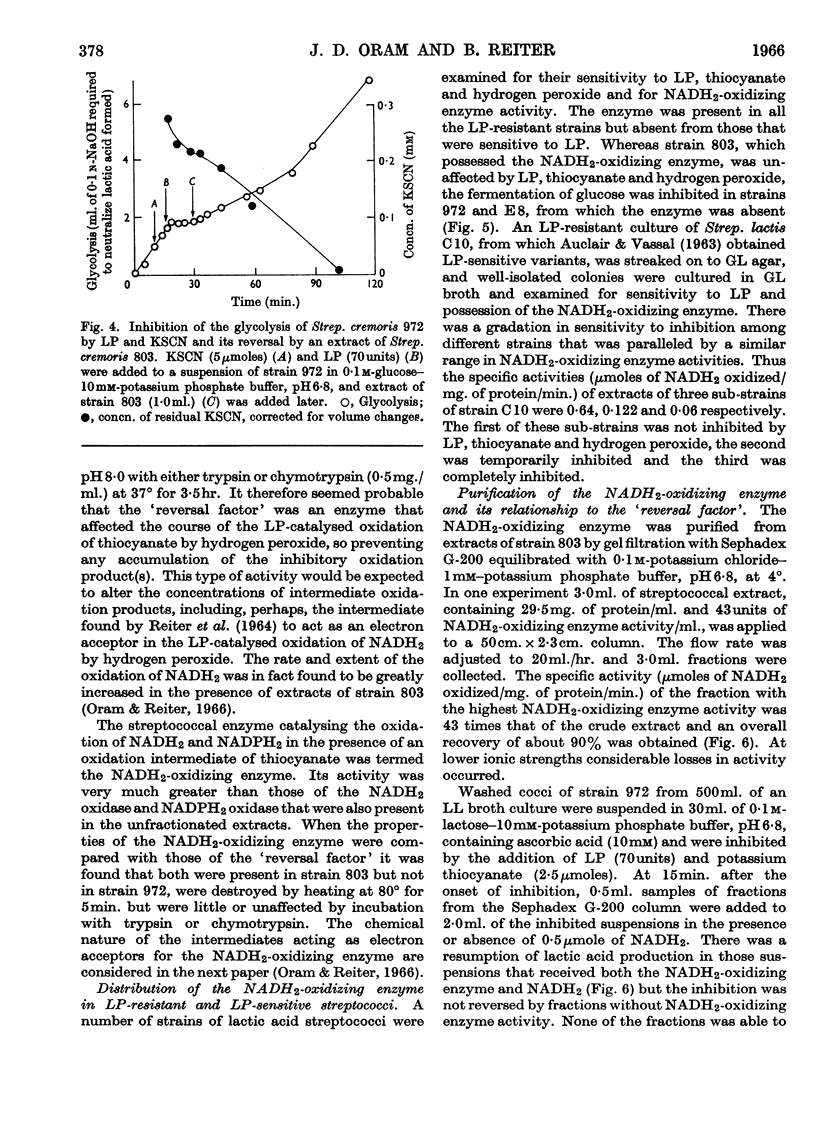
1. The growth of the lactoperoxidase-sensitive Streptococcus cremoris 972 in a synthetic medium was inhibited by lactoperoxidase and thiocyanate. The glycolysis and oxygen uptake of suspensions of Strep. cremoris 972 in glucose or lactose were also inhibited. The lactoperoxidase-resistant Strep. cremoris 803 was not inhibited under these conditions but was inhibited in the absence of a source of energy. 2. Lactoperoxidase (EC 1.11.1.7), thiocyanate and hydrogen peroxide completely inhibited the hexokinases of non-metabolizing suspensions of both strains. The inhibition was reversible, hexokinase and glycolytic activities of Strep. cremoris 972 being restored by washing the cells free from inhibitor. The aldolase and 6-phosphogluconate-dehydrogenase activities of Strep. cremoris 972 were partially inhibited but several other enzymes were unaffected. 3. The resistance of Strep. cremoris 803 to inhibition was not due to the lack of hydrogen peroxide formation, to the destruction of peroxide, to the inactivation of lactoperoxidase or to the operation of alternative pathways of carbohydrate metabolism. 4. A `reversal factor', which was partially purified from extracts of Strep. cremoris 803, reversed the inhibition of glycolysis of Strep. cremoris 972. The `reversal factor' also catalysed the oxidation of NADH2 in the presence of an intermediate oxidation product of thiocyanate and was therefore termed the NADH2-oxidizing enzyme. 5. The NADH2-oxidizing enzyme was present in lactoperoxidase-resistant streptococci but was absent from lactoperoxidase-sensitive streptococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Phage-associated lysins affecting group N and group D streptococci. J Gen Microbiol. 1965 Jul;40(1):57–70. doi: 10.1099/00221287-40-1-57. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTMANN A., AUCLAIR J. [Relation between lactenins and agglutinins of cow's milk]. Ann Inst Pasteur (Paris) 1959 Oct;97:590–596. [PubMed] [Google Scholar]
- WILSON A. T., ROSENBLUM H. The antistreptococcal property of milk. I. Some characteristics of the activity of lactenin in vitro; the effect of lactenin on hemolytic streptococci of the several serological groups. J Exp Med. 1952 Jan;95(1):25–38. doi: 10.1084/jem.95.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. T., ROSENBLUM H. The antistreptococcal property of milk. II. The effects of anaerobiosis, reducing agents, thiamine, and other chemicals on lactenin action. J Exp Med. 1952 Jan;95(1):39–50. doi: 10.1084/jem.95.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON A. T., ROSENBLUM H. The antistreptococcal property of milk. III. The role of lactenin in milk-borne epidemics; the in vivo action of lactenin. J Exp Med. 1952 Jan;95(1):51–59. doi: 10.1084/jem.95.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELDOW B. J. Studies on the antibacterial action of human saliva. III. Cofactor requirements of Lactobacillus bactericidin. J Immunol. 1963 Jan;90:12–16. [PubMed] [Google Scholar]



