Abstract
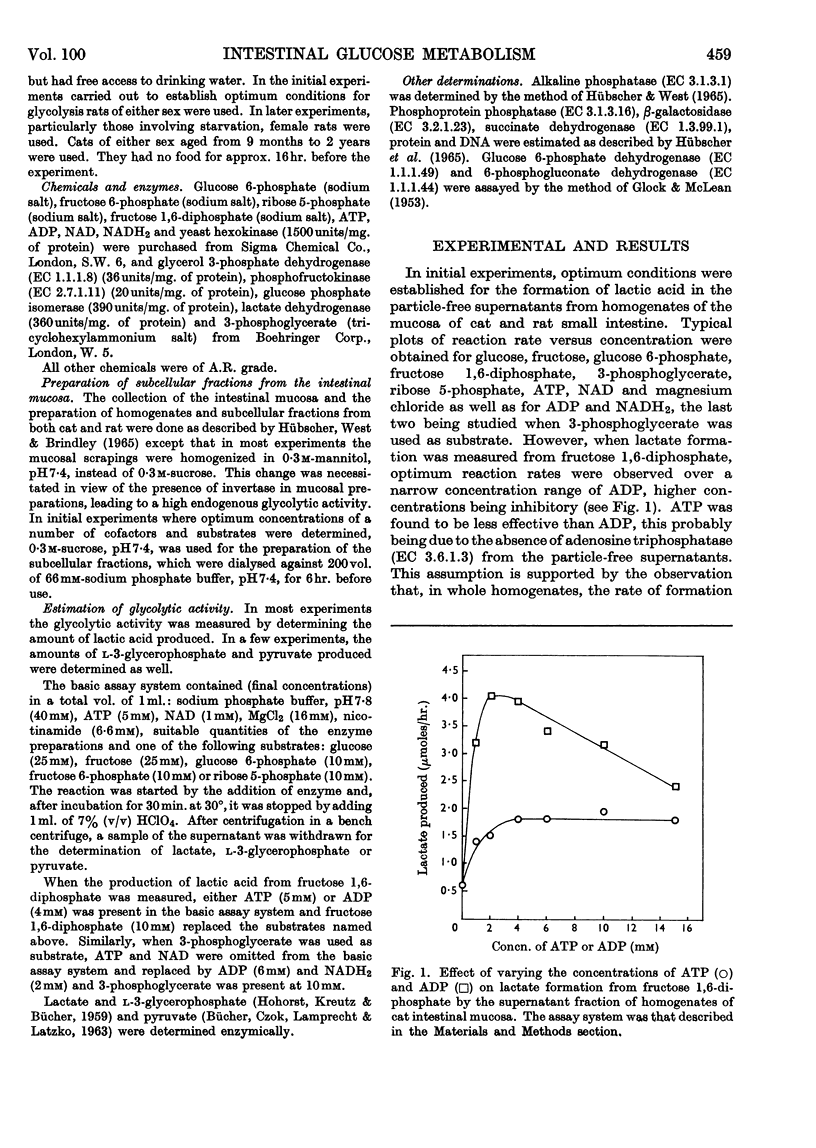
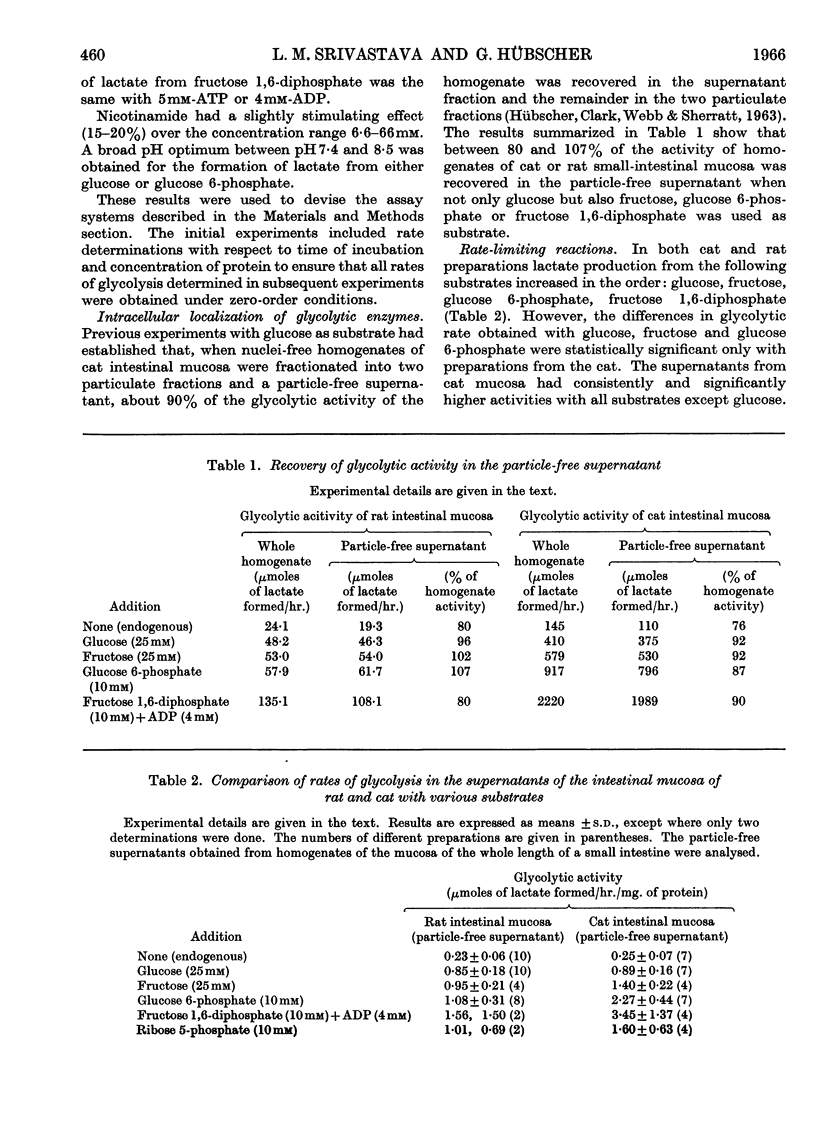
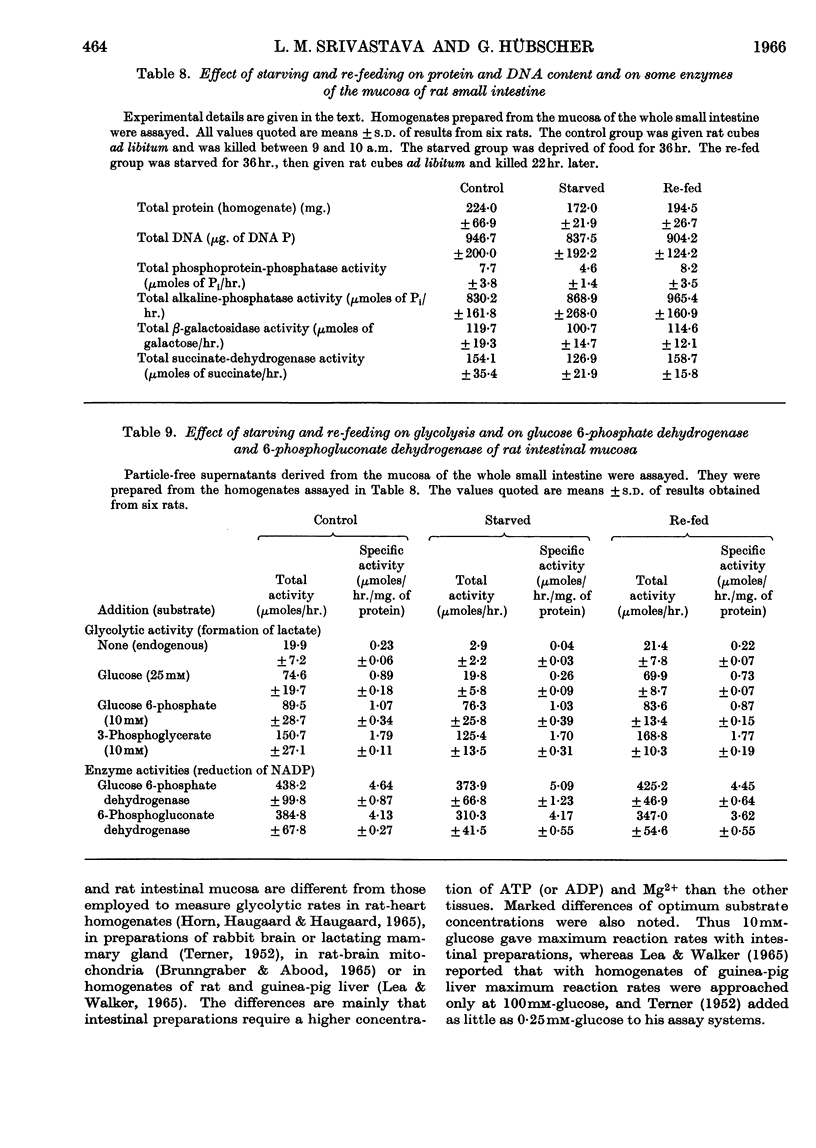
1. Lactic acid formation in supernatant fractions of homogenates of cat or rat small-intestinal mucosa was measured under optimum conditions with glucose, fructose, glucose 6-phosphate, fructose 1,6-diphosphate or 3-phosphoglycerate as substrate. 2. Between 80 and 107% of the glycolytic activity of the homogenate was recovered in these particle-free preparations when glucose, fructose, glucose 6-phosphate or fructose 1,6-diphosphate was used as substrate. 3. Evidence was obtained that hexokinase and phosphofructokinase were the rate-limiting enzymes in the initial sequence of glycolytic reactions. The limitation of rate by hexokinase was much more pronounced in preparations from the cat than in those from the rat. 4. With subcellular preparations from cat or rat small intestine lactic acid was also formed from ribose 5-phosphate and at rates similar to those observed with glucose. 5. A higher rate of glycolysis was observed with glucose 6-phosphate as substrate with preparations from the proximal half of the small intestine of the rat as compared with the distal half. 6. Mucosal preparations from rats starved for 24–48hr. exhibited only about one-quarter of the glycolytic activity of those of fed control groups. The decreased rate of formation of lactic acid from either glucose or fructose was mainly due to a decrease in the activity of hexokinase(s). The activities of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase and a number of other enzymes were not significantly decreased by starvation. 7. The results are discussed in relation to metabolic control of glycolysis in other mammalian tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F. GLUCOSEPHOSPHATE ISOMERASE OF INTESTINAL MUCOSA. Enzymologia. 1963 Jun 30;26:12–22. [PubMed] [Google Scholar]
- BRUNNGRABER E. G., ABOOD L. G. Mitochondrial glycolysis of rat brain and its relationship to the remainder of cellular glycolysis. J Biol Chem. 1960 Jul;235:1847–1853. [PubMed] [Google Scholar]
- CHAIKOFF I. L., KATZ J., KIYASU J. Y. Nature of the 14C compounds recovered in portal plasma after enteral administration of 14C-glucose. Biochim Biophys Acta. 1956 Aug;21(2):286–290. doi: 10.1016/0006-3002(56)90009-9. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z. Phosphorylation of 3-methyl glucose by hexokinase from rat's intestinal mucosa. Science. 1953 Aug 28;118(3061):253–254. doi: 10.1126/science.118.3061.253. [DOI] [PubMed] [Google Scholar]
- Clark B., Porteous J. W. The isolation and properties of epithelial-cell "ghosts" from rat small intestine. Biochem J. 1965 Aug;96(2):539–551. doi: 10.1042/bj0960539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON A. M., ISSELBACHER K. J. The esterification of palmitate-1-C14 by homogenates of intestinal mucosa. J Clin Invest. 1960 Jan;39:150–160. doi: 10.1172/JCI104014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F., Weil-Malherbe H. Metabolism of normal and tumour tissue: The metabolism of intestinal mucous membrane. Biochem J. 1941 Jan;35(1-2):7–15. doi: 10.1042/bj0350007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG V., HERS H. G. On the conversion of fructose to glucose by guinea pig intestine. Biochim Biophys Acta. 1960 Mar 11;38:427–434. doi: 10.1016/0006-3002(60)91278-6. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELE M. P. The phosphorylation and absorption of sugars in the rat. 1. Hexokinase activity in the intestinal mucosa. Biochem J. 1953 Dec;55(5):857–863. doi: 10.1042/bj0550857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESTRIN-LERNER S., SHAPIRO B. Absorption of glucose from the intestine. II. In vivo and perfusion studies. Biochim Biophys Acta. 1954 Jan;13(1):54–60. doi: 10.1016/0006-3002(54)90270-x. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Horn R. S., Haugaard E. S., Haugaard N. The mechanism of the inhibition of glycolysis by oxygen in rat heart homogenate. Biochim Biophys Acta. 1965 Jun 22;99(3):549–552. doi: 10.1016/s0926-6593(65)80210-7. [DOI] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGMAN G. I., KARDAMAN S., HABER J. AMINE LEVELS, MONOAMINE OXIDASE AND DOPA-DECARBOXYLASE ACTIVITIES IN THE GASTRO-INTESTINAL TRACT OF THE RAT. Life Sci. 1964 Nov;3:1355–1360. doi: 10.1016/0024-3205(64)90056-6. [DOI] [PubMed] [Google Scholar]
- KOLDOVSKY O., CHYTIL F. POSTNATAL DEVELOPMENT OF BETA-GALACTOSIDASE ACTIVITY IN THE SMALL INTESTINE OF THE RAT. EFFECT OF ADRENALECTOMY AND DIET. Biochem J. 1965 Jan;94:266–270. doi: 10.1042/bj0940266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEA M. A., WALKER D. G. FACTORS AFFECTING HEPATIC GLYCOLYSIS AND SOME CHANGES THAT OCCUR DURING DEVELOPMENT. Biochem J. 1965 Mar;94:655–665. doi: 10.1042/bj0940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG C. Studies involving enzymic phosphorylation. I. The hexokinase activity of rat tissues. Biochem J. 1952 Jan;50(3):407–415. doi: 10.1042/bj0500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONG C. Studies involving enzymic phosphorylation. II. Changes in the hexokinase activity of the small intestine of rats caused by feeding different diets. Biochem J. 1953 Jan;53(1):7–12. doi: 10.1042/bj0530007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALHOTRA O. P., PHILIP G. HYDROLYTIC ENZYMES OF MAMMALIAN INTESTINES. II. DISTRIBUTION OF HYDROLYTIC ENZYMES IN DOG, GUINEA-PIG, SQUIRREL, ALBINO RAT AND RABBIT INTESTINES. Indian J Med Res. 1965 May;53:410–416. [PubMed] [Google Scholar]
- NEWEY H., SMYTH D. H., WHALER B. C. The absorption of glucose by the in vitro intestinal preparation. J Physiol. 1955 Jul 28;129(1):1–11. doi: 10.1113/jphysiol.1955.sp005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWARA M. T. Hexokinase and phosphatase activities of intestinal mucosa following thyroidectomy and thyroid administration. Endocrinology. 1961 May;68:850–854. doi: 10.1210/endo-68-5-850. [DOI] [PubMed] [Google Scholar]
- SCHAPIRA F. [Fructose-1-phosphoaldolase activity of mammalian tissues. I. Distribution of fructose-1-phosphoaldolase activity in mammalian tissues]. Bull Soc Chim Biol (Paris) 1961;43:1357–1365. [PubMed] [Google Scholar]
- SOLS A. The hexokinase activity of the intestinal mucosa. Biochim Biophys Acta. 1956 Jan;19(1):144–152. doi: 10.1016/0006-3002(56)90396-1. [DOI] [PubMed] [Google Scholar]
- TERNER C. Anaerobic and aerobic glycolysis in lactating mammary gland and in nervous tissue. Biochem J. 1952 Oct;52(2):229–237. doi: 10.1042/bj0520229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLAR-PALASI C., LARNER J. Levels of activity of the enzymes of the glycogen cycle in rat tissues. Arch Biochem Biophys. 1960 Feb;86:270–273. doi: 10.1016/0003-9861(60)90417-3. [DOI] [PubMed] [Google Scholar]
- WILSON T. H. Concentration gradients of lactate, hydrogen and some other ions across the intestine in vitro. Biochem J. 1954 Mar;56(3):521–527. doi: 10.1042/bj0560521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H. The role of lactic acid production in glucose absorption from the intestine. J Biol Chem. 1956 Oct;222(2):751–763. [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. Metabolic activity of the small intestine of the rat and golden hamster (Mesocricetus auratus). J Physiol. 1954 Jan;123(1):126–130. doi: 10.1113/jphysiol.1954.sp005037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R. RATE-LIMITING FACTORS IN GLYCOLYSIS AND INORGANIC ORTHOPHOSPHATE TRANSPORT IN RAT LIVER AND KIDNEY SLICES. J Biol Chem. 1965 Jun;240:2373–2381. [PubMed] [Google Scholar]
- Weil-Malherbe H. Observations on tissue glycolysis. Biochem J. 1938 Dec;32(12):2257–2275. doi: 10.1042/bj0322257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de TORRONTEGUI Hexokinase activity of intestinal mucosa after feeding different diets. Biochim Biophys Acta. 1961 Jun 10;50:164–165. doi: 10.1016/0006-3002(61)91073-3. [DOI] [PubMed] [Google Scholar]


