Abstract
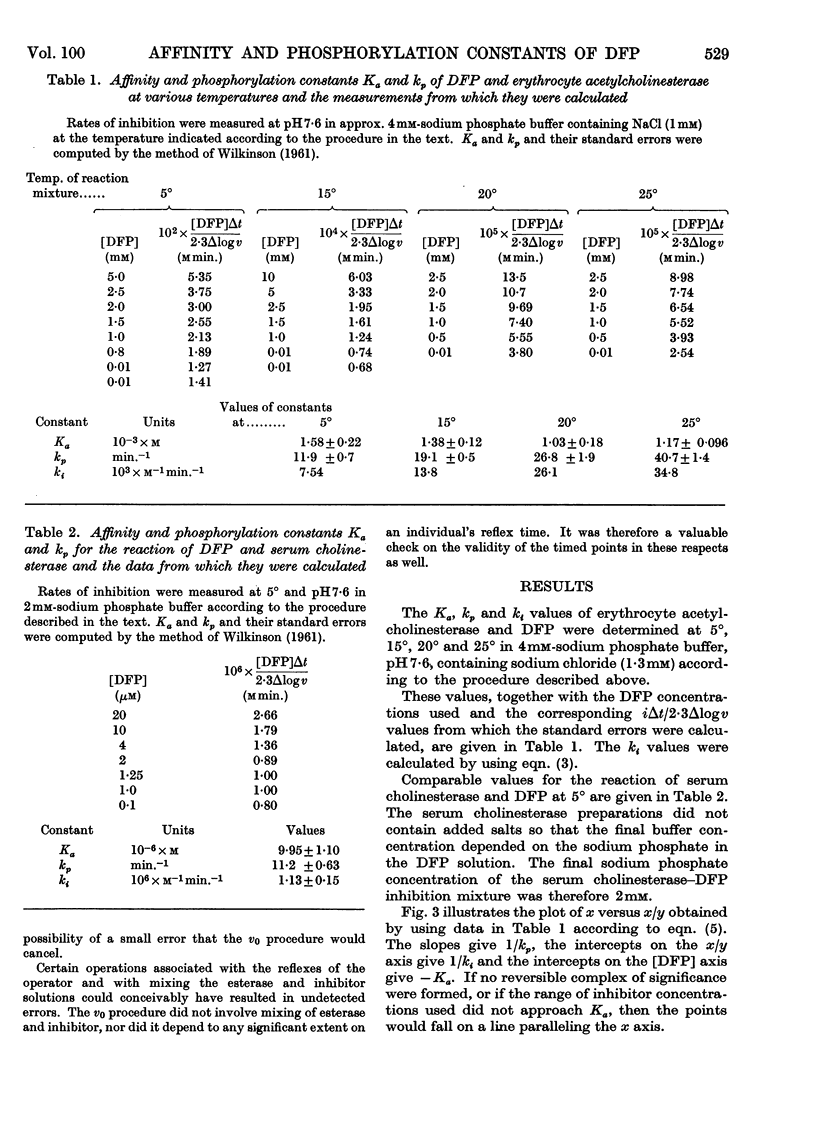
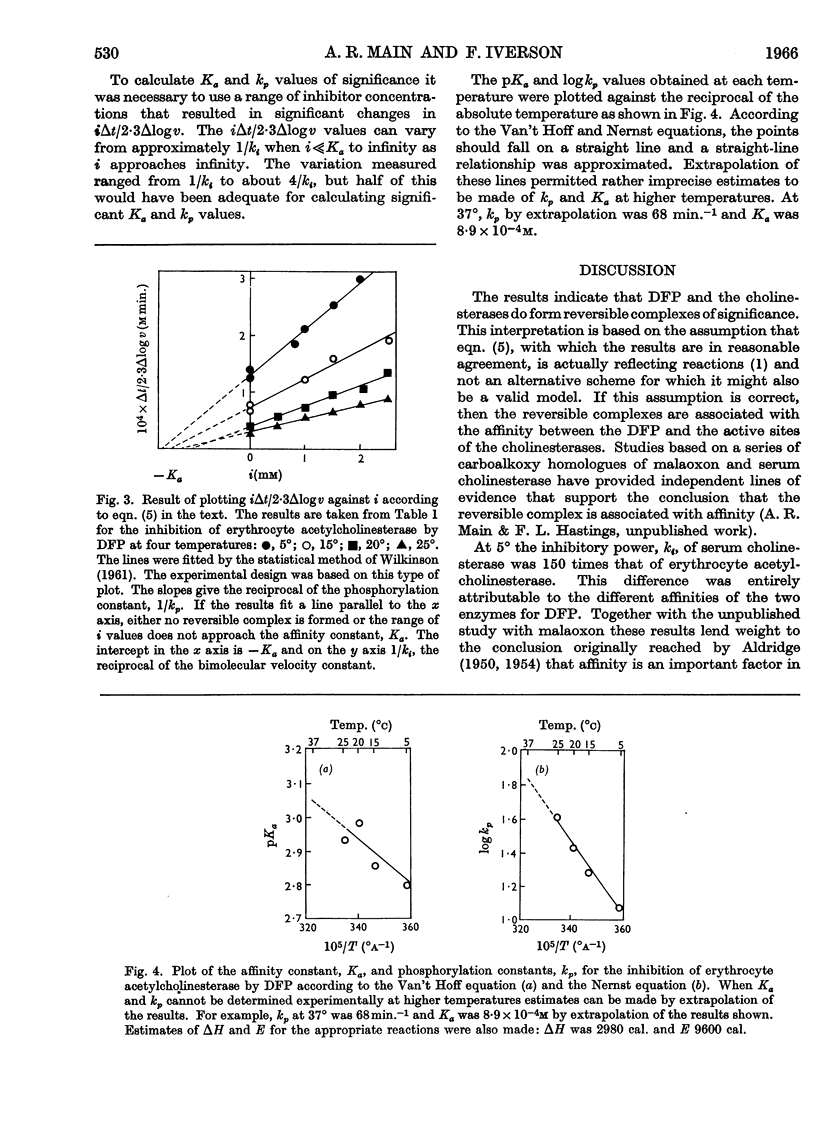
1. A procedure is described for determining the affinity constant Ka and the phosphorylation constant kp for the inhibition by di-isopropyl phosphorofluoridate of erythrocyte acetylcholinesterase and serum cholinesterase. The procedure depends on the use of a specially designed reaction vessel with which incubation times as short as 1·2sec. could be obtained at any convenient temperature. 2. The Ka of acetylcholinesterase decreased from 1·58 (±0·22)×10−3m at 5° to 1·17 (±0·10)×10−3m at 25° and the associated change in enthalpy was 2980 cal. 3. The kp of acetylcholinesterase increased from 11·9 (±0·7)min.−1 at 5° to 40·7 (±1·4)min.−1 at 25°, indicating an activational energy of 9600 cal. The change in entropy associated with Ka was 23·5 cal. degree−1 at 25°. 4. At 5°, the Ka and kp of serum cholinesterase were 9·95 (±1·10)×10−6m and 11·2 (±0·63)min.−1 respectively. 5. The 150-fold difference in the inhibitory power of di-isopropyl phosphorofluoridate for the two cholinesterases was attributed entirely to differences in affinity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Some properties of specific cholinesterase with particular reference to the mechanism of inhibition by diethyl p-nitrophenyl thiophosphate (E 605) and analogues. Biochem J. 1950 Apr;46(4):451–460. doi: 10.1042/bj0460451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD A. M., FAHRNEY D. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. II. FORMATION AND REACTIONS OF PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1964 Jun;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. THE inhibition of chymotrypsin by diethyl p-nitrophenyl phosphate. Biochem J. 1952 Mar;50(5):672–678. doi: 10.1042/bj0500672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- MAIN A. R. AFFINITY AND PHOSPHORYLATION CONSTANTS FOR THE INHIBITION OF ESTERASES BY ORGANOPHOSPHATES. Science. 1964 May 22;144(3621):992–993. doi: 10.1126/science.144.3621.992. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


