Abstract
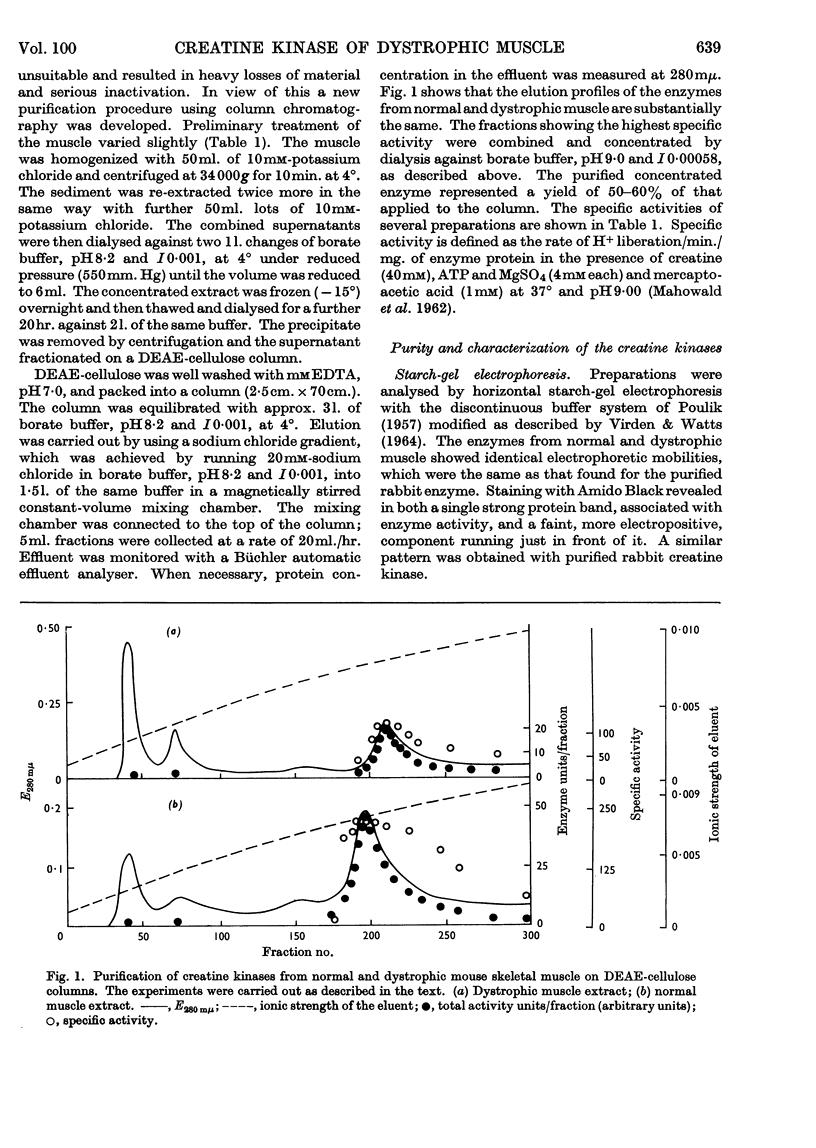
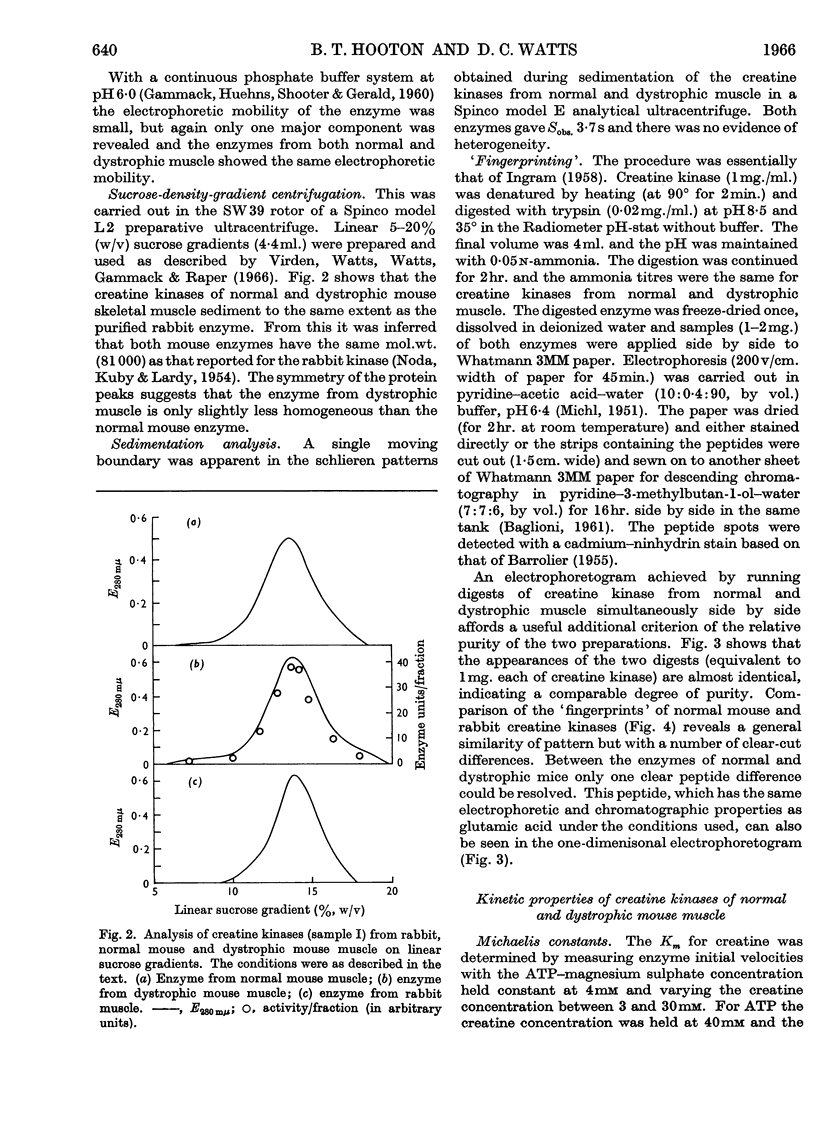
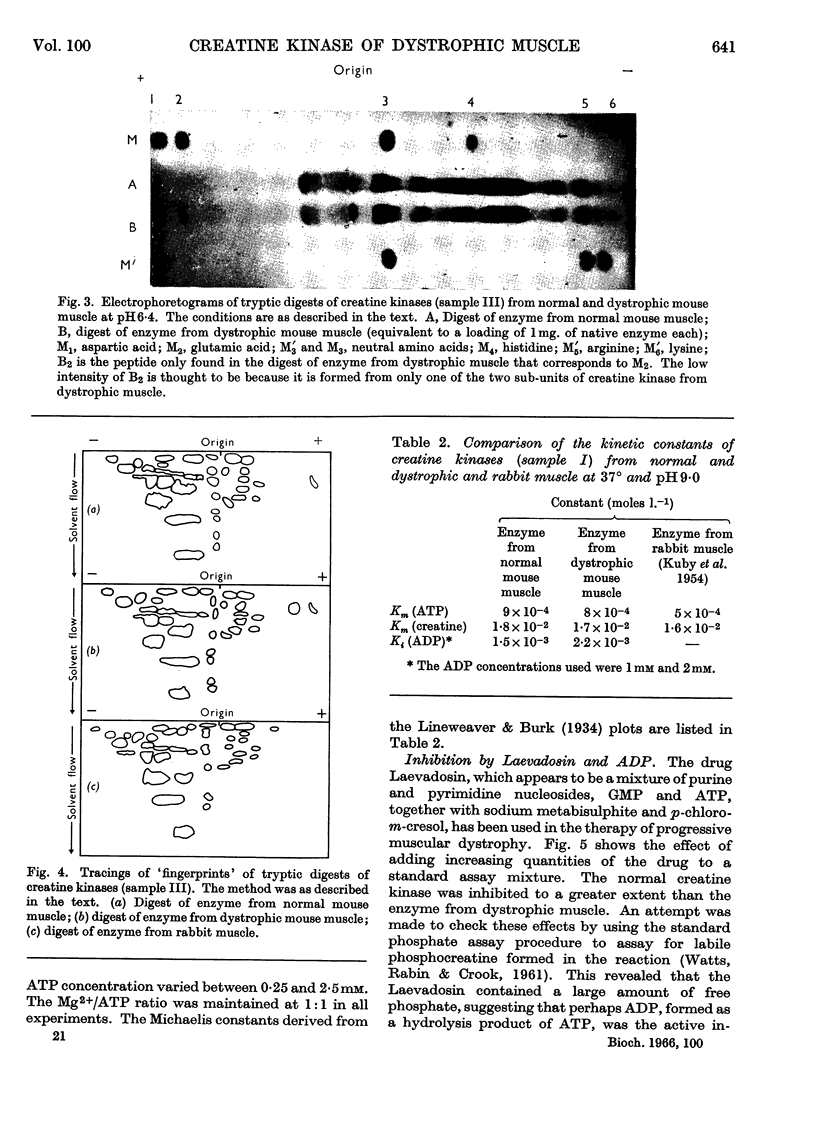
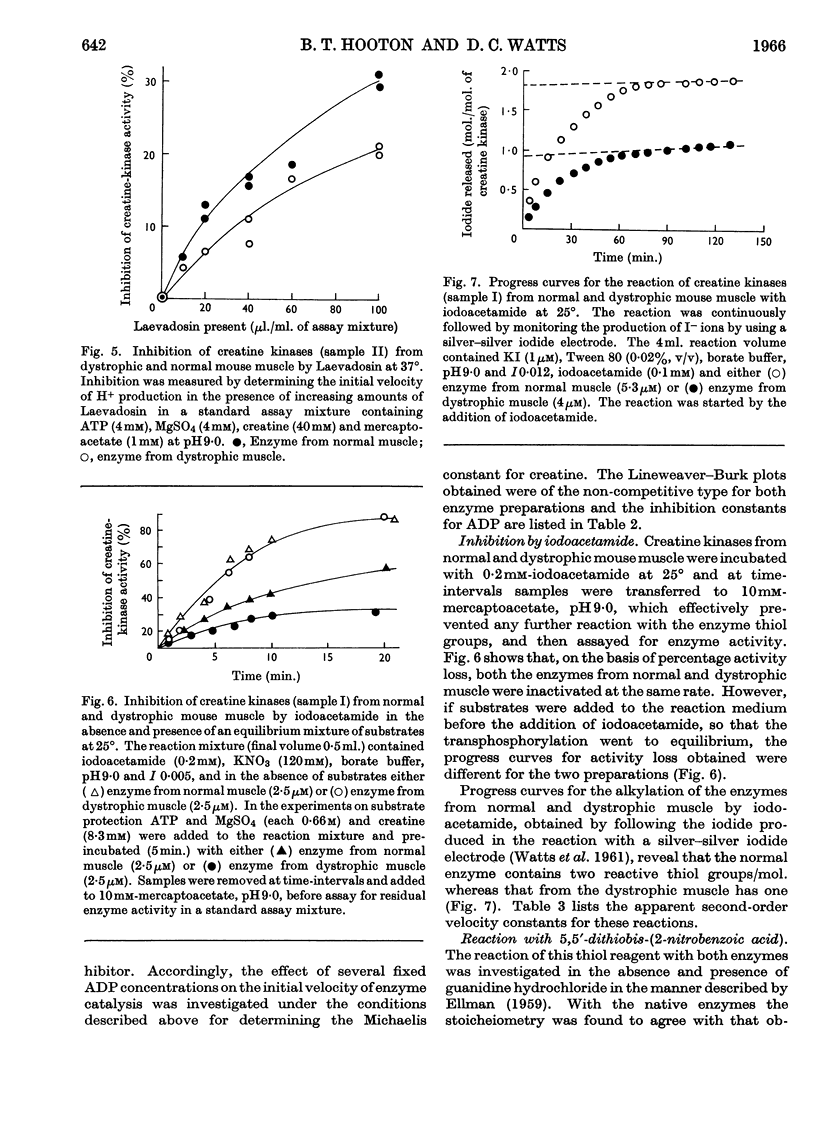
1. A column procedure for the purification of creatine kinase from normal and dystrophic mouse muscle is described. 2. The native enzymes are indistinguishable by various physical criteria and have mol.wt. about 80000. 3. The purified enzyme from dystrophic muscle is only half as active as the normal, contains only one thiol group readily alkylated by iodoacetamide instead of two and has one less free thiol group/mol. 4. Michaelis constants for MgATP and creatine are the same for both preparations. 5. The inhibitor constant for ADP at pH9·0 is different in the two enzymes and this may account for the different degrees of inhibition observed in vitro with the drug Laevadosin. 6. The enzyme from dystrophic muscle is protected by an equilibrium mixture of substrates against inhibition by iodoacetamide to a greater extent than the normal enzyme. 7. `Fingerprinting' suggests one peptide difference between creatine kinases from normal and dystrophic muscle. 8. The possibility that this finding represents the primary lesion in dystrophy is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAGLIONI C. An improved method for the fingerprinting of human hemoglobin. Biochim Biophys Acta. 1961 Apr 1;48:392–396. doi: 10.1016/0006-3002(61)90490-5. [DOI] [PubMed] [Google Scholar]
- CARLSON F. D., SIGER A. The mechanochemistry of muscular contraction. I. The isometric twitch. J Gen Physiol. 1960 Sep;44:33–60. doi: 10.1085/jgp.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO A. K., HASLETT W. L., JENDEN D. J. A titrimetric method for the determination of creating phosphokinase. Biochem J. 1960 Apr;75:115–119. doi: 10.1042/bj0750115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER A. C., MILLDR J. R. Progressive muscular dystrophy: a review. Rev Can Biol. 1962 Sep-Dec;21:337–351. [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. Creatine kinase: evidence for a dimeric structure. Biochem Biophys Res Commun. 1965 Nov 22;21(4):346–353. doi: 10.1016/0006-291x(65)90200-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- GAMMACK D. B., HUEHNS E. R., SHOOTER E. M., GERALD P. S. Identification of the abnormal polypeptide chain of hemoglobin G-Ib. J Mol Biol. 1960 Dec;2:372–378. doi: 10.1016/s0022-2836(60)80048-4. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Abnormal human haemoglobins. I. The comparison of normal human and sickle-cell haemoglobins by fingerprinting. Biochim Biophys Acta. 1958 Jun;28(3):539–545. doi: 10.1016/0006-3002(58)90516-x. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- MAHOWALD T. A., NOLTMANN E. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. III. Inhibition reactions. J Biol Chem. 1962 May;237:1535–1548. [PubMed] [Google Scholar]
- McCaman M. W. Enzyme studies of skeletal muscle in mice with hereditary muscular dystrophy. Am J Physiol. 1963 Nov;205(5):897–901. doi: 10.1152/ajplegacy.1963.205.5.897. [DOI] [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. II. Homogeneity and physicochemical properties. J Biol Chem. 1954 Jul;209(1):203–210. [PubMed] [Google Scholar]
- NOLTMANN E. A., MAHOWALD T. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. II. Amino acid composition of adenosine triphosphate-creatine transphosphorylase. J Biol Chem. 1962 Apr;237:1146–1154. [PubMed] [Google Scholar]
- OLSON O. E., KUBY S. A. STUDIES ON ADENOSINE TRIPHOSPHATE-ADENOSINE TRIPHOSPHATE TRANSPHOSPHORYLASES. V. CARBOXYL-TERMINAL SEQUENCES OF ADENOSINE TRIPHOSPHATE-CREATINE TRANSPHOSPHORYLASE AND OF ADENOSINE 5'-PHOSPHATE TRANSPHOSPHORYLASE (MYOKINASE). J Biol Chem. 1964 Feb;239:460–467. [PubMed] [Google Scholar]
- PEARCE J. M., GUBBAY S. S., HARDY J., PENNINGTON R. J., NEWELL D. J., WALTON J. N. LAEVADOSIN IN TREATMENT OF DUCHENNE TYPE OF MUSCULAR DYSTROPHY: PRELIMINARY RESULTS OF A DOUBLE-BLIND CONTROLLED TRIAL. Br Med J. 1964 Oct 10;2(5414):915–917. doi: 10.1136/bmj.2.5414.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. Creatine phosphokinase and the enzymic and contractile properties of the isolated myofibril. Biochem J. 1954 Jul;57(3):427–434. doi: 10.1042/bj0570427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Pennington R. J. Biochemistry of dystrophic muscle. 2. Some enzyme changes in dystrophic mouse muscle. Biochem J. 1963 Jul;88(1):64–68. doi: 10.1042/bj0880064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOLLER M., FINEBERG R. A. STUDIES OF MYOSIN IN HEREDITARY MUSCULAR DYSTROPHY IN MICE. J Clin Invest. 1965 Apr;44:615–622. doi: 10.1172/JCI105174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON A. R., EVELEIGH J. W., MILES B. J. AMINO-ACID SEQUENCE AROUND THE REACTIVE THIOL GROUPS OF ADENOSINE TRIPHOSPHATE--CREATINE PHOSPHOTRANSFERASE. Nature. 1964 Jul 18;203:267–269. doi: 10.1038/203267a0. [DOI] [PubMed] [Google Scholar]
- VIRDEN R., WATTS D. C. THE DISTRIBUTION OF GUANIDINE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES AND ADENOSINE TRIPHOSPHATASE IN ANIMALS FROM SEVERAL PHYLA. Comp Biochem Physiol. 1964 Oct;13:161–177. doi: 10.1016/0010-406x(64)90202-6. [DOI] [PubMed] [Google Scholar]
- Virden R., Watts D. C., Watts R. L., Gammack D. B., Raper J. H. Adenosine 5'-triphosphate-arginine phosphotransferase from lobster muscle. Molecular weight. Biochem J. 1966 Apr;99(1):155–158. doi: 10.1042/bj0990155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R., CROOK E. M. The reaction of iodoacetate and iodoacetamide with proteins as determined with a silver/silver iodide electrode. Biochim Biophys Acta. 1961 Apr 1;48:380–388. doi: 10.1016/0006-3002(61)90488-7. [DOI] [PubMed] [Google Scholar]
- Watts D. C., Rabin B. R., Crook E. M. The number of catalytic sites in creatine phosphokinase as determined by a study of its reactive sulphydryl groups. Biochem J. 1962 Mar;82(3):412–417. doi: 10.1042/bj0820412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI K., MASE R. Coupled reaction of creatine kinase and myosin A-adenosine triphosphatase. J Biol Chem. 1962 Feb;237:397–403. [PubMed] [Google Scholar]
- YAGI K., NODA L. Phosphate transfer to myofibrils by ATP-creatine transphosphorylase. Biochim Biophys Acta. 1960 Sep 23;43:249–259. doi: 10.1016/0006-3002(60)90435-2. [DOI] [PubMed] [Google Scholar]



