Abstract
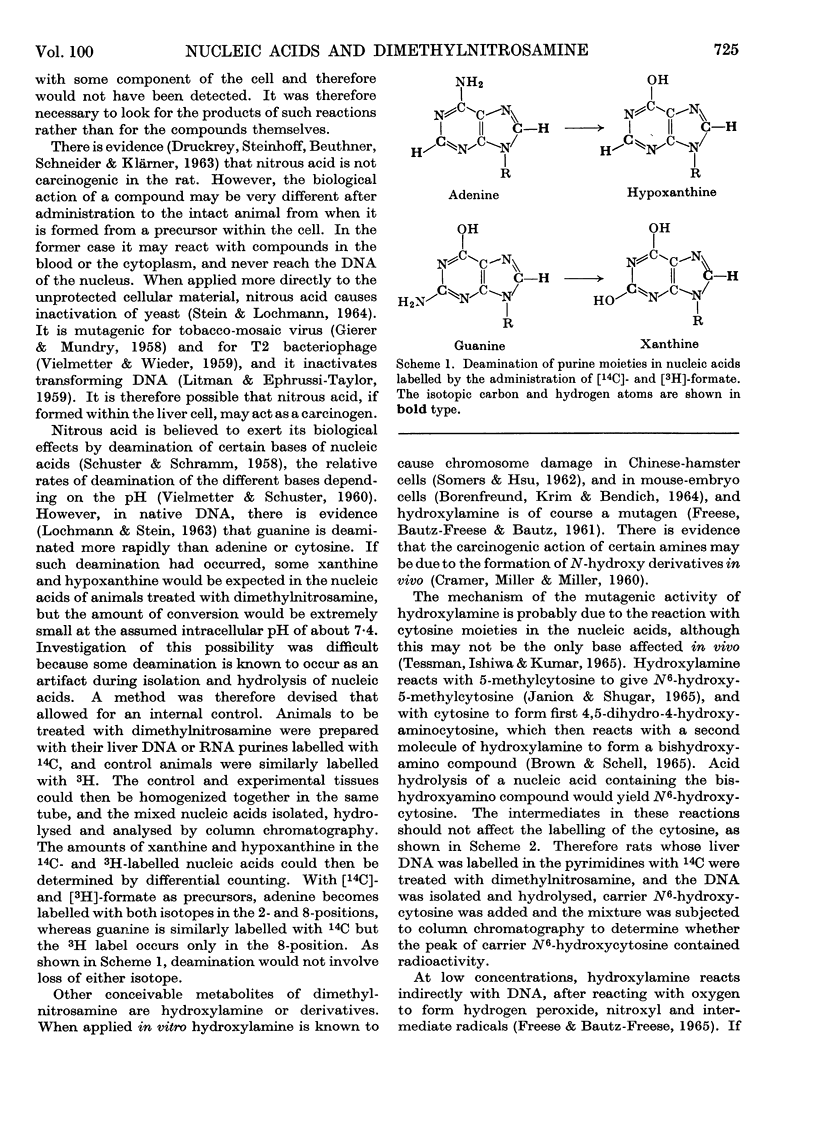
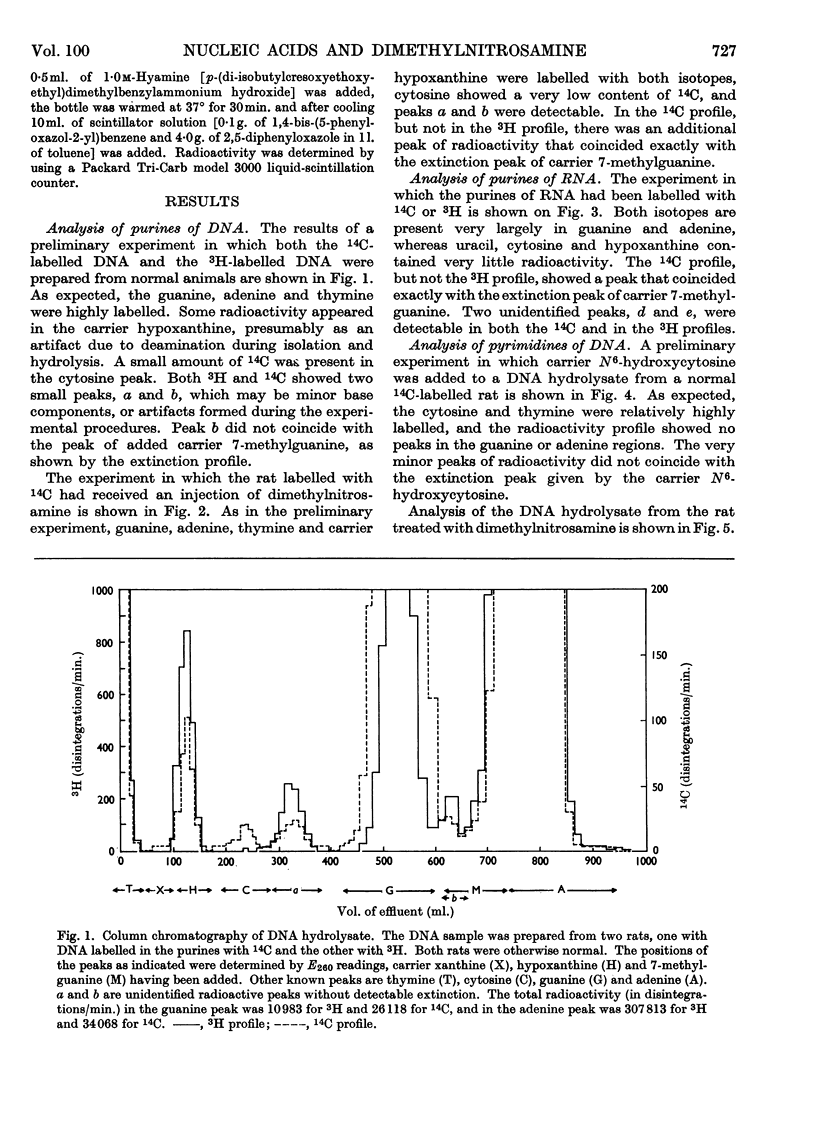
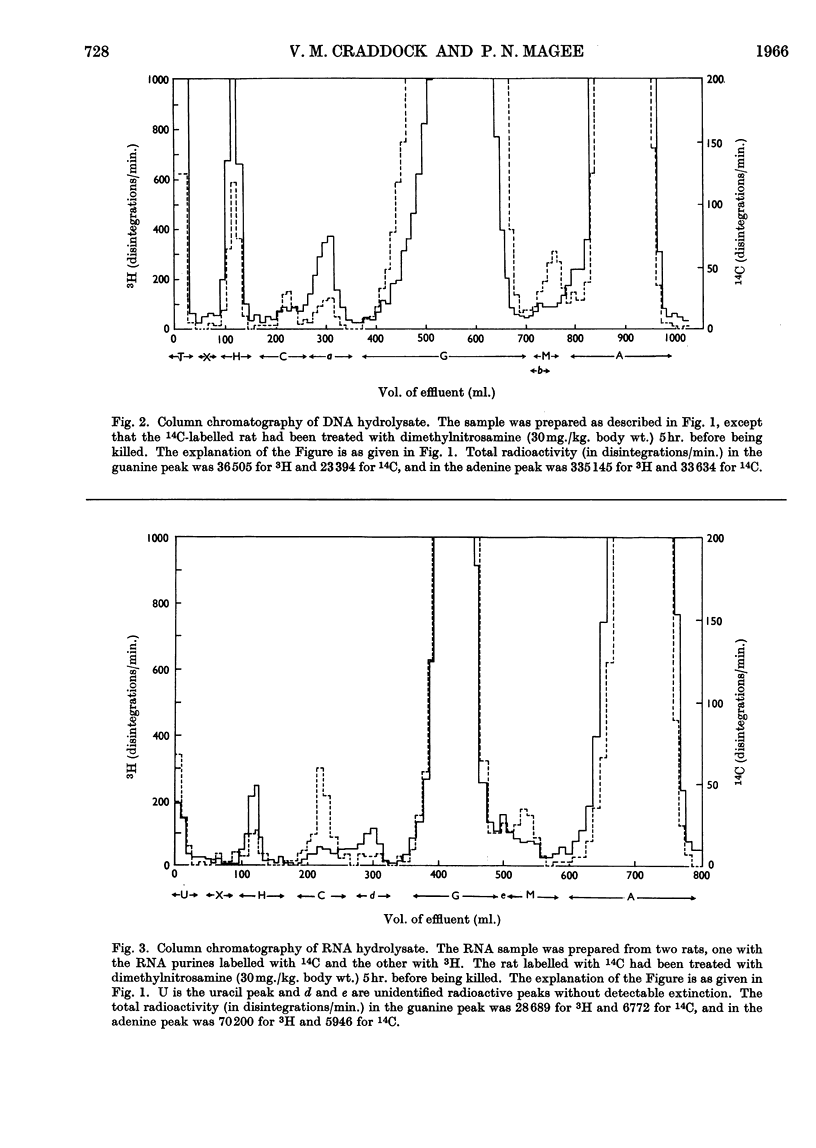
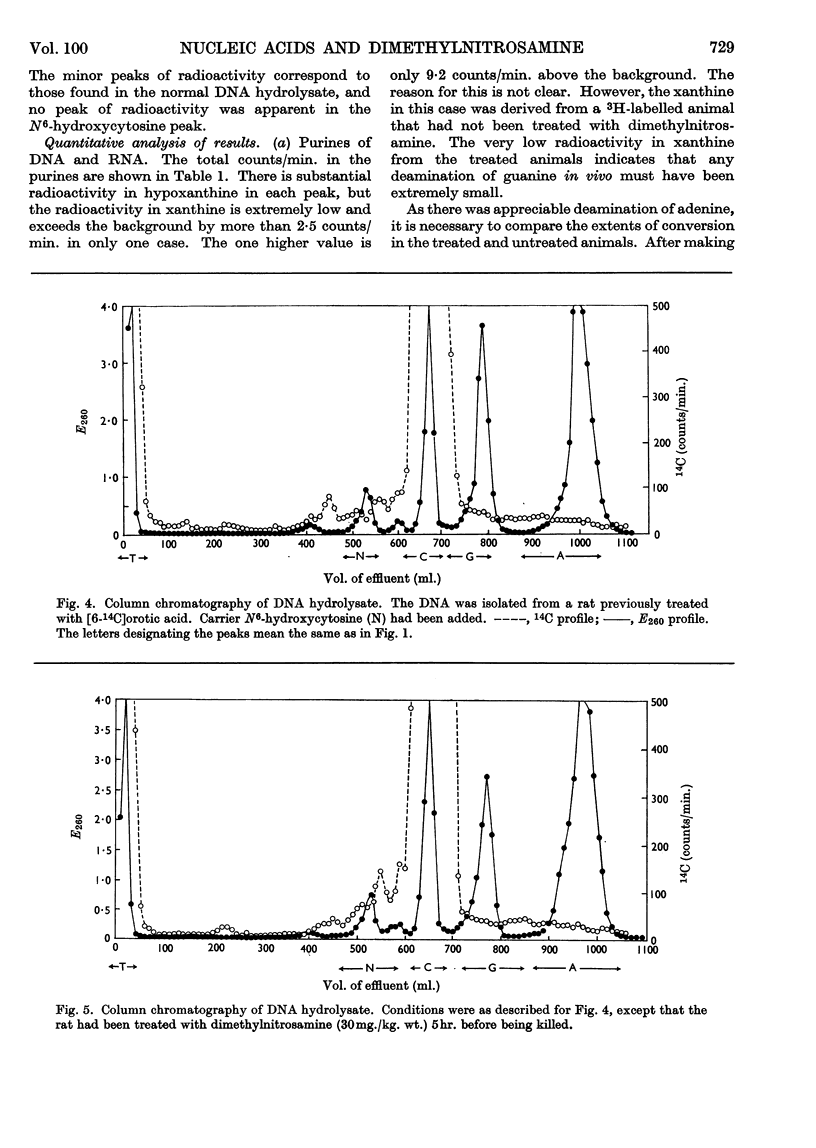
Dimethylnitrosamine is metabolized to form an alkylating intermediate, which may have significance for its carcinogenic action. However, certain other compounds that are known to be highly mutagenic, including nitrous acid and hydroxylamine, might also be formed. Owing to the general reactivity of these compounds, it would be difficult to detect their formation in the intact animal. Instead, the nucleic acids of carcinogen-treated animals were examined for products of reaction with nitrous acid and hydroxylamine, i.e. for deamination of adenine and guanine, and formation of N6-hydroxycytosine, respectively. A double-labelling technique was used to detect very small amounts of the abnormal bases. The purine moieties of DNA in adult rat liver were labelled either with 14C or with 3H, by treating the neonatal animals with [14C]formate or with [3H]formate, and then allowing a period for normal growth. During this time, in liver, the labels were largely lost by metabolic turnover from cell components other than DNA. The pyrimidine moieties in DNA were labelled by treating the neonatal animals with [14C]orotate. The purine constituents of RNA of adult rat liver were labelled by repeated administration of [14C]- or [3H]-formate to the adult rats. The [14C]nucleic acid-labelled rat could then be treated with the carcinogen, and the [3H]nucleic acid-labelled animal could be used as a control. By this means the experimental and control tissues could be homogenized together in a single preparation, and the nucleic acids from the two tissues could be isolated, hydrolysed and analysed in a single sample. It was therefore possible to have an internal control for artifacts due to changes taking place in the nucleic acid bases during the experimental procedures. With this technique, the formation in vivo of 7-methylguanine in rat-liver DNA and RNA after administration of dimethylnitrosamine was confirmed, and no evidence was found for the formation of xanthine, hypoxanthine, N6-hydroxycytosine, or any other abnormal base.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORENFREUND E., KRIM M., BENDICH A. CHROMOSOMAL ABERRATIONS INDUCED BY HYPONITRITE AND HYDROXYLAMINE DERIVATIVES. J Natl Cancer Inst. 1964 Mar;32:667–679. [PubMed] [Google Scholar]
- BOYLAND E. Mutagens. Pharmacol Rev. 1954 Sep;6(3):345–364. [PubMed] [Google Scholar]
- BROWN D. M., SCHELL P. NUCLEOTIDES. 48. THE REACTION OF HYDROXYLAMINE WITH CYTOSINE AND RELATED COMPOUNDS. J Chem Soc. 1965 Jan;47:208–215. [PubMed] [Google Scholar]
- CRADDOCK V. M., MAGEE P. N. REACTION OF THE CARCINOGEN DIMETHYLNITROSAMINE WITH NUCLEIC ACIDS IN VIVO. Biochem J. 1963 Oct;89:32–37. doi: 10.1042/bj0890032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRADDOCK V. M. REACTION OF THE CARCINOGEN DIMETHYLNITROSAMINE WITH PROTEINS AND WITH THIOL COMPOUNDS IN THE INTACT ANIMAL. Biochem J. 1965 Feb;94:323–330. doi: 10.1042/bj0940323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMER J. W., MILLER J. A., MILLER E. C. N-Hydroxylation: A new metabolic reaction observed in the rat with the carcinogen 2-acetylaminofluorene. J Biol Chem. 1960 Mar;235:885–888. [PubMed] [Google Scholar]
- DUNN A., STRAHS S. A COMPARISON OF 3H- AND 14C-GLUCOSE METABOLISM IN THE INTACT RAT. Nature. 1965 Feb 13;205:705–706. doi: 10.1038/205705a0. [DOI] [PubMed] [Google Scholar]
- FREESE E., BAUTZ-FREESE E., BAUTZ E. Hydroxylamine as a mutagenic and inactivating agent. J Mol Biol. 1961 Apr;3:133–143. doi: 10.1016/s0022-2836(61)80040-5. [DOI] [PubMed] [Google Scholar]
- GIERER A., MUNDRY K. W. Production of mutants of tobacco mosaic virus by chemical alteration of its ribonucleic acid in vitro. Nature. 1958 Nov 22;182(4647):1457–1458. doi: 10.1038/1821457a0. [DOI] [PubMed] [Google Scholar]
- HEATH D. F., DUTTON A. The detection of metabolic products from dimethylnitrosamine in rats and mice. Biochem J. 1958 Dec;70(4):619–626. doi: 10.1042/bj0700619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANION C., SHUGAR D. REACTION OF HYDROXYLAMINE WITH 5-SUBSTITUTED CYTOSINES. Biochem Biophys Res Commun. 1965 Feb 17;18:617–622. doi: 10.1016/0006-291x(65)90800-4. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. Ribonucleic acids. II. Improved preparation of rat-liver ribonucleic acid. Biochim Biophys Acta. 1962 Apr 2;55:545–546. doi: 10.1016/0006-3002(62)90988-5. [DOI] [PubMed] [Google Scholar]
- LITMAN R. M., EPHRUSSI-TAYLOR H. [Inactivation and mutation of the genetic factors of the desoxyribonucleic acid of pneumococcus by ultraviolet light and by nitrous acid]. C R Hebd Seances Acad Sci. 1959 Aug 10;249:838–840. [PubMed] [Google Scholar]
- MAGEE P. N., BARNES J. M. The experimental production of tumours in the rat by dimethylnitrosamine (N-nitroso dimethylamine). Acta Unio Int Contra Cancrum. 1959;15(1):187–190. [PubMed] [Google Scholar]
- MAGEE P. N., BARNES J. M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br J Cancer. 1956 Mar;10(1):114–122. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGEE P. N., FARBER E. Toxic liver injury and carcinogenesis. Methylation of rat-liver nucleic acids by dimethylnitrosamine in vivo. Biochem J. 1962 Apr;83:114–124. doi: 10.1042/bj0830114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. A., MILLER E. C. METABOLISM OF DRUGS IN RELATION OF CARCINOGENICITY. Ann N Y Acad Sci. 1965 Mar 12;123:125–140. doi: 10.1111/j.1749-6632.1965.tb12250.x. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., HEIDELBERGER C. METABOLIC REGULATORY CIRCUITS AND CARCINOGENESIS. Cancer Res. 1963 Nov;23:1694–1700. [PubMed] [Google Scholar]
- SCHUSTER H., SCHRAMM G. Bestimmung der biologisch wirksamen Einheit in der Ribosenucleinsäure des Tabakmosaikvirus auf chemischem Wege. Z Naturforsch B. 1958 Nov;13B(11):697–704. [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERS C. E., HSU T. C. Chromosome damage induced by hydroxylamine in mammalian cells. Proc Natl Acad Sci U S A. 1962 Jun 15;48:937–943. doi: 10.1073/pnas.48.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN W., LOCHMANN E. R. DIE WIRKUNG VON NATRIUMNITRIT AUF NUCLEINSAEUREBASEN UND IHR VERHAELTNIS ZUR INAKTIVIERUNG VON HEFEZELLEN. II. DIE INAKTIVIERUNG VON SACCHAROMYCES-ZELLEN VERSCHIEDENEN PLOIDIEGRADES. Z Naturforsch B. 1964 Jul;19:625–633. [PubMed] [Google Scholar]
- STRONG L. C. Genetic concept for the origin of cancer: historical review. Ann N Y Acad Sci. 1958 Sep 30;71(6):810–838. doi: 10.1111/j.1749-6632.1958.tb46811.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R., Borek E. THE SPECIES VARIATION OF RNA METHYLASE. Proc Natl Acad Sci U S A. 1963 Apr;49(4):529–533. doi: 10.1073/pnas.49.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSMAN I., ISHIWA H., KUMAR S. MUTAGENIC EFFECTS OF HYDROXYLAMINE IN VIVO. Science. 1965 Apr 23;148(3669):507–508. doi: 10.1126/science.148.3669.507. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The nucleic acids of some insect viruses. J Gen Physiol. 1952 Nov;36(2):201–205. doi: 10.1085/jgp.36.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]


