Abstract
The ability of two different flow cytometers, the Microcyte (Optoflow) and the PAS-III (Partec), to differentiate sporangia of the late-blight pathogen Phytophthora infestans from other potential airborne particles was compared. With the PAS-III, light scatter and intrinsic fluorescence parameters could be used to differentiate sporangia from conidia of Alternaria or Botrytis spp., rust urediniospores, and pollen of grasses and plantain. Differentiation between P. infestans sporangia and powdery mildew conidia was not possible by these two methods but, when combined with analytical rules evolved by genetic programming methods, could be achieved after staining with the fluorescent brightener Calcofluor white M2R. The potential application of these techniques to the prediction of late-blight epiphytotics in the field is discussed.
Current methods for predicting the occurrence of late-blight of potato (Phytophthora infestans) rely on climatic modeling to identify conditions conducive to pathogen reproduction and thus to disease spread (5). Such methods can provide considerable savings to growers, as well as environmental benefits. For United Kingdom potato production, calendar spraying is normal practice, with sprays commencing when the crop canopy has closed and continuing at 7- to 10-day intervals until crop desiccation or harvest. Use of forecasting models can reduce the number of required sprays by allowing a delay in the first fungicide application and/or an extension of the interval between subsequent sprays (e.g., references 25 and 28).
However, such methods are not always reliable in all conditions. Evaluation of five forecasting systems at different sites in England and Wales from 1994 to 1997 indicated that their predictions differed from one another and that the effectiveness of all the models varied from year to year. They were at their least effective in a low-risk year, when they recommended spraying even though no blight occurred (N. J. Bradshaw, M. C. Taylor, and N. V. Hardwick, Abstr. Int. Cong. Plant Pathol., abstr. 3:1.21, 1998). Geographical location may also affect the performance of forecasting models: differences in climate, as well as in local blight populations, may mean that systems which function well in their country of origin may not perform as well elsewhere (14, 24, 28).
One important factor that is not taken into account by forecasting models is the amount of inoculum present in the immediate environment of the crop. Although there is evidence for a soil-borne inoculum of oospores in other European countries (15, 39), in the United Kingdom it appears that asexual, wind-borne sporangia remain the most important source of late-blight inoculum (J. P. Day, R. C. Shattock, and D. S. Shaw, unpublished data). Use of volumetric spore traps within potato fields, with identification and enumeration of sporangia carried out under the microscope, has yielded variable results. Schlenzig et al. (36) detected inoculum in the air only after the first diseased plants were visible in the field, and they concluded that sampling of the air was no more effective than a visual check of the crop in terms of identifying the start of the epidemic. In contrast, Bugiani et al. (6, 7) found that one or two peaks in sporangia concentration in the air (10 to 20 sporangia per m3) preceded the first observed symptoms, and they used these data to confirm the predictions of climatic modeling. It is evident that the sensitivity of such methods would be greatly enhanced if an increased rate of sampling could be coupled with a means of identifying and enumerating the P. infestans sporangia automatically.
Flow cytometry is a well-proven technique that allows cells, spores, and other particulates to be analyzed individually (12, 16, 33, 37, 44). Particles are interrogated optically and, for each particle, measurements of several cellular parameters are recorded: these normally include forward light scatter, orthogonal (side) light scatter, and one or more fluorescence parameters. Particles may show intrinsic autofluorescence; for example, the autofluorescence of the photosynthetic pigments of microalgae and cyanobacteria, combined with light scatter measurements, facilitates differentiation of different species by flow cytometry (e.g., references 10, 40, 42, and 43). In other cases, fluorescent dyes are needed to differentiate different microbes (12, 34, 42). For example, forward light scatter, wide-angle light scatter, and DNA fluorescence (stained with two fluorescent dyes) have been used to differentiate spores of different species of basidiomycete fungi (1).
Flow cytometry has many advantages with regard to the detection of single particles, but the expense of traditional instrumentation, and its large size and comparatively high-power requirements, pose disadvantages for an in-field system. The Microcyte flow cytometer (Optoflow AS; Oslo, Norway [12, 20]) offers a portable system, capable of battery operation and with relatively small dimensions (330 by 430 by 160 mm). Sheath fluid is recycled internally, and the optical components are mounted in a solid aluminum block requiring no user adjustments.
The Microcyte would present an ideal in-field system, if directly linked to a high-volume air sampler, but it has limitations including, for example, the relatively small number of parameters recorded and the presentation of data as a population histogram only rather than in list mode (37), which records and presents data individually from each particle. In the experiments reported here, the Microcyte was compared with the PAS-III particle analyzing system (Partec GmbH, Münster, Germany), a larger, benchtop system with dual-wavelength excitation and up to six optical parameters. The ability of both instruments to differentiate P. infestans sporangia from pollens and fungal spores was assessed by using both light scatter and fluorescence parameters, with a view toward the detection and enumeration of P. infestans inoculum against a background of some other common constituents of the air spora. The most effective discrimination of P. infestans sporangia from the other particles tested was achieved with the PAS-III and relied upon a combination of the autofluorescence of certain particles (especially pollen and rust urediniospores) and the fluorescent brightener Calcofluor white M2R.
MATERIALS AND METHODS
Source and preparation of fungal spores and pollen grains.
An A1 mating-type isolate of P. infestans, designated 96.69, was used for all experiments. This culture had been isolated in 1996 as part of a United Kingdom late-blight survey (also funded by the Ministry of Agriculture, Fisheries, and Food) carried out at the University of Wales, Bangor, United Kingdom, and was known to belong to the most commonly occurring clonal lineage in England and Wales (Day et al., unpublished). Cultures were maintained for long-term storage on rye A agar (8) supplemented with RAN (50 μg of rifamycin, 50 μg of ampicillin, and 100 μg of nystatin per ml [Sigma Aldrich Co., Ltd., Poole, United Kingdom]). Large numbers of sporangia for experiments were obtained by first culturing them on unamended rye A agar for ca. 7 days and then using a sporangial suspension obtained from these plates to inoculate detached potato leaves (cv. Home Guard, commercial suppliers). These were incubated in plastic salad boxes containing a double layer of paper towel above a damp cloth to maintain humidity. After 6 to 7 days of incubation at 18°C, with 18 h of illumination daily, leaves were washed in distilled water to obtain sporangial suspensions.
Cultures of Botrytis cinerea and Alternaria alternata were isolated from senescing potato tissue from the glass house. Cultures of Penicillium chrysogenum (NRRL 1951) were obtained from the laboratory culture collection. These cultures were maintained on potato dextrose agar (Lab M; Topley House, Bury, United Kingdom), and conidia were harvested by washing the plates with distilled water. Plant materials infected with powdery mildew (Blumeria graminis var. hordei) and with rust fungi (oat crown rust [Puccinia coronata f. sp.avenae] and wheat brown rust [Puccinia recondita f. sp. tritici]) were provided by Tim Carver and Elwyn Jones of the Institute of Grassland and Environmental Research (IGER), Aberystwyth, United Kingdom. Conidia and urediniospores of mildew and rusts were harvested by agitating leaves in 50 ml of distilled water containing a single drop of Tween 80. Pollen of Poa annua (annual meadow grass) was obtained from plants grown in the glass house from seed donated by Ian Thomas of the Genetic Resources Unit at the IGER. Other grass, plantain, and pine pollens were obtained from plants on campus at the University of Wales, Aberystwyth. In each case, harvested cones and/or flowers were allowed to release pollen overnight.
Suspensions of biological particles were filtered through a single layer of muslin (average pore size, ca. 0.5 mm) before being placed in the flow cytometer. Spores or sporangia obtained from leaves were“washed” twice by allowing them to settle out of suspension and then removing and replacing the top layers of the suspension. For most analyses, particles were at a concentration of ca. 105 ml−1. For some experiments, particles were fixed or killed by the addition of ethanol to the medium (50% final volume).
Staining of spores.
The following dyes were obtained from Molecular Probes Europe BV: 1,1′,3,3,3′,3′-hexamethylindodicarbo-cyanine iodide [DiIC1(5)], 3,3′-dipropylthiadicarbo-cyanine iodide [DiSC3(5)], TO-PRO-3 iodide, and a range of SYTO dyes (SYTO 17, 59, 60, 61, 62, 63, and 64). Nile Blue A was obtained from Electron Microscopy Sciences, Fort Washington, Pa., and Calcofluor white M2R (Fluorescent Brightener 28; Tinopal UNPA-GX) was from Sigma Aldrich Co., Ltd. Stock solutions were dissolved in dimethyl sulfoxide except for Nile Blue and Calcofluor white, which were made up in distilled water. After addition of dye, samples were routinely kept in the dark for 15 min at room temperature before being measured in the flow cytometer.
Flow cytometry.
Flow cytometric analyses were performed by using (i) the Microcyte portable flow cytometer (Optoflow AS) purchased from Aber Instruments, Ltd. (Aberystwyth, Wales, United Kingdom) with a 635-nm laser diode as light source and avalanche photodiode detectors for forward light scatter (636 ± 5 nm) and red fluorescence (650 to 800 nm) or (ii) the Partec PAS-III particle analyzing system (Partec GmbH), which was equipped with a 488-nm argon-ion laser, a 633-nm helium-neon laser, a 100-W Hg arc lamp, and photomultiplier tube detectors for six optical parameters. For most experiments, data were collected with respect to forward scatter (FSC), side scatter (SSC), and four fluorescence parameters: FL1 (green; 515 to 560 nm), FL2 (orange; 575 to 605 nm), FL3 (red; >645 nm), and FL4 (red; 665 to 690 nm). For experiments with Calcofluor white, the PAS-III was operated with the 488-nm argon-ion laser and the mercury arc lamp as a UV light source. In this configuration, fluorescence from the UV excitation was recorded (at 450 to 460 nm) instead of SSC.
Both instruments were set to record on a logarithmic intensity scale. The following reference standards were used: 6.0-μm AlignFlow Plus Flow Cytometry Alignment beads (Molecular Probes Europe BV, Leiden, The Netherlands) and 10.5-, 41.5-, and 66.0-μm-diameter Uniform Microspheres (Bangs Laboratories, Inc., Fishers, Ind.). Data analysis was carried out by techniques of multiple gating (FCS Express; De Novo Software, Thornhill, Ontario, Canada) and genetic programming. Genetic programming (2, 29–32) is a technique that allows the evolution of simple, interpretable rules from complex datasets. We have been applying it to a wide variety of problems in spectroscopy (17, 21, 26, 41, 45) and elsewhere (18). For the present program we used the program Gmax-bio (Aber Genomic Computing, Aberystwyth, Wales) with default settings for the population size (1,000) and recombination rate (80%) and a mixture of numerical and logical operators. Hexavariate data (FSC, SSC/UV, FL1, FL2, FL3, and FL4) were acquired from 9,000 particles (variously Phytophthora sporangia; spores of Alternaria, Blumeria, crown rust, and brown rust; and pollen of Poa annua). Data for Phytophthora were encoded with a“1”; data for the remaining particles were encoded with a “ 0”. The program evolved rules, and the program that finally evolved was checked against a test (holdout) set of data that it had not seen (see reference 27).
RESULTS
Light-scatter detection of P. infestans sporangia with the Microcyte.
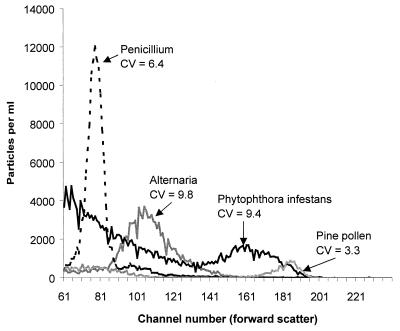
FSC histograms obtained from the Microcyte could be used to differentiate some large populations of particles from others (e.g., Fig. 1). However, most of the types of pollen tested in this instrument showed FSC histograms that were extremely similar to those of P. infestans sporangia. Fungal conidia normally showed less FSC than the sporangia, but even here there was some “overlap” between the histograms. Since the software recorded data only as histograms, it was not possible to generate two-dimensional plots of FSC against fluorescence for individual particles as a further aid for particle differentiation.
FIG. 1.
Individual light-scatter plots, overlaid, for sporangia of P. infestans, conidia of Penicillium chrysogenum and Alternaria spp., and pollen of a Pinus sp. (mean of four samples each) recorded in the Microcyte. The plot for P. infestans also shows a large number of small background particles, since these samples were collected from infected leaves. The CV ([standard deviation × 100]/mean channel number) was determined only for the range of channel numbers containing the sporangia or spores of interest.
Peak channel numbers (PCN) for FSC measured in the Microcyte were plotted against the actual dimensions of the particles (particle width, particle length, particle volume, and their logarithms) as measured under the microscope. The size of particles was measured on three separate occasions, with 30 particles measured each time, and the overall mean was plotted on the graphs. Different datum points on the graphs correspond to experiments on different days; the variation observed between readings for the same particle types on different days may have been due to slight variations in particle size and/or metabolic state, as well as to differences in the performance of the flow cytometer. Particle volume was estimated by using the assumption that particles could be regarded as cylinders with hemispherical ends.R2 values close to 1.0 (see Fig. 2 legend) indicated that the relationship between FSC and particle width and/or length or volume was relatively close to linear in the case of the latex beads; linear correlation was less strong for the biological particles. The strongest linear relationships were found for particle width (Fig. 2) or, to a lesser extent, volume, while particle length gave the weakest correlation. The presence of 50% ethanol (which fixed or killed the biological particles) had no significant effect on peak channel number for any of the biological particle types tested (P < 0.05; single-factor analysis of variance [analyses not shown]). A relatively larger scatter signal was obtained from the latex beads than from most biological particles of similar size (cf. reference 11). P. infestans sporangia and zoospores gave a larger light-scatter signal, relative to their actual size, than the other biological particles. In the Microcyte, sporangia appeared to be similar in size to pollen, in spite of being smaller in their true width.
FIG. 2.
Correlation between FSC and log (particle width) for standard beads (6.0, 10.5, 41.5, and 66.0 μm), P. infestans sporangia, other fungal spores, and pollen analyzed in the Microcyte. Separate datum points indicate the peak channel number for samples analyzed on different days. Linear least-squares best-fit lines were calculated in MS Excel and are shown with correlation coefficient R2 = 1 − (SSE/SST), where SSE = ∑(Yj −Ŷj)2 and SST = (∑Yj2) − (∑ Yj)2/n.
Light-scatter detection of P. infestans sporangia with the PAS-III.
For most of the particles tested, FSC histograms obtained for P. infestans sporangia in the PAS-III were more sharply defined than those observed in the Microcyte, i.e., they showed a lower coefficient of variation (CV). For example, a CV of ca. 5.0 was usual for live P. infestans sporangia measured in the PAS-III (ranging from 3.6 to 7.3).
PCN for both FSC and SSC (i.e., orthogonal or “side” scatter) were plotted against particle width, length, and volume and against their logarithms. R2 values of >0.9 were obtained for FSC plotted against width, log(width), and log(volume) for the“standard” beads (R2 was only 0.85 for FSC plotted against volume), but no such correlations were observed for the biological particles (e.g., Fig. 3). Viable P. infestans sporangia and zoospores,Blumeria and Botrytis conidia, and grass pollen (Agrostis or Poa) showed relatively high FSC, for their size; rust urediniospores, Alternaria conidia, killed P. infestans sporangia, and Plantago pollen showed relatively low FSC. The correlation of SSC with particle size was similar to, or better than, that of FSC (e.g., Fig. 4). The SSC of rust urediniospores was similar to that of the beads. SSC of viable sporangia,Blumeria conidia, and Plantago or Poa pollen was slightly higher than that of the beads, while the SSC of killed sporangia and Agrostis pollen was considerably higher. Zoospores and Alternaria or Botrytis conidia showed relatively low SSC, for their size.
FIG. 3.
Relationship between FSC and log (particle width) for standard beads, P. infestans sporangia and zoospores, other fungal spores, and pollen analyzed in the PAS-III. Separate datum points indicate the PCN for samples analyzed on different days. Linear least-squares best-fit lines were calculated in MS Excel (see Fig. 2).
FIG. 4.
Correlation between SSC and log (particle width) for standard beads, P. infestans sporangia and zoospores, other fungal spores, and pollen analyzed in the PAS-III. Separate datum points indicate the PCN for samples analyzed on different days. Linear least-squares best-fit lines were calculated in MS Excel (see Fig. 2).
The ratio between SSC and FSC for Alternaria conidia and rust urediniospores was similar to that of the standard beads (Fig. 5). Other (viable) spores or sporangia showed lower SSC values relative to FSC, while the SSC/FSC ratio for killed sporangia and for grass or plantain pollens was relatively high. It is also clear from Fig. 5 that Blumeria conidia cannot be differentiated from sporangia of P. infestans by using FSC and SSC alone.
FIG. 5.
Correlation between SSC and FSC for standard beads,P. infestans sporangia and zoospores, other fungal spores, and pollen analyzed in the PAS-III. A logarithmic least-squares best-fit line was calculated for beads only; R2 was calculated in MS Excel by using a transformed regression model.
Intrinsic fluorescence.
It was noted with both the Microcyte and the PAS-III that certain biological particles exhibited red“autofluorescence,” notably pollen, rust urediniospores,Blumeria conidia (particularly nonviable conidia) and, to a lesser degree, P. infestans sporangia (particularly those that had been killed by exposure to 50% ethanol). In the PAS-III, both the fluorescence signals and the SSC signals are collected at an angle orthogonal both to the direction of the laser and to that of the sample fluid flow. Fluorescence signals for each of the four wavelength“windows” collected were therefore plotted against the SSC for a variety of pollen grains, fungal spores, and beads to determine which of them exhibited intrinsic fluorescence. Good correlation was observed between green fluorescence and SSC for both beads and biological particles, suggesting that the green fluorescence recorded represented no more than imperfect blocking by the filters. For orange or red fluorescence, however, rust urediniospores showed higher fluorescence than expected, as did pollen, killed sporangia of P. infestans and, to a lesser degree, viable P. infestans sporangia and Blumeria conidia (e.g., Fig. 6). It appears therefore that these particles exhibit various levels of orange and red intrinsic fluorescence, which could be used as a further aid to differentiation.
FIG. 6.
Correlation between orange autofluorescence (575 to 605 nm) and SSC for standard beads, P. infestans sporangia and zoospores, other fungal spores, and pollen analyzed in the PAS-III. We excluded 6-μm beads since these were fluorescent standards and could not be shown on the same scale. Agrostis pollen also could not be shown on the same scale (orange fluorescence [FL] MCN > 3,500). MCN, median channel number (considered to be more reliable than the peak channel number for asymmetric or irregular distributions which were often found for fluorescence intensity). The linear least-squares best-fit line for beads was calculated in MS Excel (see Fig. 2).
Differentiation of P. infestans from other fungal spores using light scatter and autofluorescence.
By using a combination of FSC, SSC, and measurements of autofluorescence, P. infestans sporangia could readily be differentiated, in the PAS-III, from all of the tested biological particles except for Blumeria conidia. Multiple gating was used to exclude particles that did not conform to the same FSC, SSC, and fluorescence parameters as the sporangia. Data were first gated by using polygonal regions drawn from plots of FSC versus SSC drawn separately for each spore or pollen type to exclude “background” particles (particularly important for sporangia isolated from leaves and for pollens). Additional regions were drawn from data plots obtained from P. infestans sporangia, to include only particles whose FSC, SSC, and fluorescence intensity matched that of the sporangia. Gating with all four fluorescence parameters was compared to gating with only two fluorescence parameters (Table 1).
TABLE 1.
Effectiveness of multiple gating, using FSC, SSC, and two or four FL parameters measured in the PAS-III, for differentiation of P. infestans sporangia from other fungal spores and from pollen grains in the absence of fluorescent dye
| Source | % Particles falling within gates defined for P. infestans sporangiaa
|
|
|---|---|---|
| Gated with R1 and R2 and R3b | Gated with R1 and R2c | |
| Alternaria sp. | 0, 0, 6 | 0, 0, 4 |
| Blumeria sp. | 2, 10, 10, 17, 41 | 0, 2, 38, 40, 61 |
| Botrytis sp. | 0, 0 | 0, 0 |
| Crown rust | 0, 3 | 1, 3 |
| Brown rust | 0, 2 | 0, 2 |
| Agrostis sp. | 0 | 0 |
| Plantago sp. | 3 | 1 |
| Poa sp. | 1, 2, 2 | 0, 1, 5 |
| P. infestans sporangia | 39, 50, 51, 53, 57, 71, 71, 74, 74, 81, 84, 84 | 61, 66, 68, 69, 75, 79, 80, 81, 81, 85, 87, 88 |
Figures separated by commas indicate experiments on different days.
R1, R2, and R3 were polygonal regions drawn from two-dimensional density plots of FSC versus SSC, green fluorescence versus red (> 645 nm) fluorescence and orange fluorescence versus red (665 to 690 nm) fluorescence, respectively, derived from data for sporangial suspensions. In the case of the other spores and pollens, data were first gated by using polygonal regions drawn from plots of FSC versus SSC for the individual data sets, in order to remove “background” particles.
R1 and R2 were polygonal regions drawn from two-dimensional density plots of FSC versus SSC and green fluorescence versus orange fluorescence, respectively, derived from data for sporangial suspensions. Data for other spores and pollens were first gated to remove background as described above.
The percentage of false positives (Table 1) was the percentage of particles in suspensions of pollen grains or other fungal spores which fell within the regions defined for P. infestans sporangia analyzed on the same day. For most of the spores (Alternaria, Botrytis, and rusts) and pollens (Agrostis, Plantago, and Poa) tested, there were fewer than 3% (or, rarely, 6%) false positives; however, for suspensions of Blumeria conidia, the number of false positives was extremely variable, ranging from 2% to as high as 61% on different experimental days. Use of all six parameters for gating (i.e., inclusion of the two red fluorescence parameters) was not considered to improve detection since it (i) did not consistently decrease the number of false positives (compared with the number observed when gating with FSC, SSC, green fluorescence, and orange fluorescence only) and (ii) always decreased the number of true positives (i.e., the number of particles in the sporangial suspensions identified as sporangia).
Evaluation of red fluorescent dyes.
Sporangia and zoospores of P. infestans stained readily with nuclear dyes (TO-PRO-3, SYTO dyes), with the lipid-staining dye Nile Blue, and with the membrane energization dyes DiSC3(5) and DiI1C(5). These dyes were less effective at staining spores of Penicillium, Alternaria, and Botrytis spp. and could therefore be used as an aid to differentiate these spores from P. infestans sporangia, either with the Microcyte or the PAS-III. However, these dyes were less effective at differentiating sporangia from the species of pollen tested.
For DiSC3(5) and Nile Blue, 1 μM was found to be an effective concentration to stain sporangia adequately, while 10 μM SYTO-17 was required to give consistent staining; the same concentrations were also effective for most of the other particles tested. TO-PRO-3 was less effective (for all particles except Poa pollen), even at 10 μM. The clearest differentiation between sporangia and fungal spores was achieved with DiSC3(5), but only at the higher concentration (10 μM); this dye could not be used to differentiate sporangia from Poa pollen, however. Differentiation of sporangia from pollen (but not from fungal spores) was more effective with SYTO-17 or TO-PRO-3 or with low concentrations (100 nM) of DiSC3(5).
The multiple gating analysis was repeated for sporangia, and other spores or pollens stained with these dyes, at concentrations of 1 or 10μM. The number of false positives was reduced to <0.1% in all cases for Alternaria with any of the four dyes at either concentration. However, for each of the four red dyes tested the number of false positives for Blumeria and rusts was either similar to or greater than the number that had been obtained in the absence of fluorescent dye. In the case of pollen, also, the number of false positives was either similar to or much higher than the numbers observed in the absence of dye. Detection of P. infestans sporangia against a background of spores and pollen was not, therefore, improved by any of the red dyes tested.
Staining and fluorescence detection of P. infestans sporangia and other spores or pollens in the PAS-III with Calcofluor white.
The fluorescent brightener Calcofluor white, used in the PAS-III with a UV lamp and a 488-nm laser, was effective at staining sporangia and Poa pollen at 10 μM, 100 μM, and 1 mM (Fig. 7). Staining of other fungal spores, including Blumeria, was much less effective, even at the highest concentration. UV fluorescence of Poa pollen and sporangia was similar in the presence of Calcofluor at 10 or 100 μM, but sporangia stained more effectively than pollen with 1 mM Calcofluor. Multiple gating with FSC, UV, and green and orange fluorescence parameters indicated that 10 or 100 μM Calcofluor was effective for distinguishing sporangia from any of the other fungal spores tested (including Blumeria) and also from Poa pollen (Table 2). Comparison of multiple gating with different parameters indicated that red fluorescence did not provide any further aid to differentiation by this method, since red fluorescence tended to be more variable than the other measured parameters, making it more difficult to select the regions for gating (data for red fluorescence were included in the genetic programming analysis, however). Since SSC results were not collected when the PAS-III was set up to record UV, the initial gating step to remove “background” particles from the fungal spore and pollen suspensions was carried out with regions drawn from plots of FSC versus green fluorescence. The same initial gating step was also used for data used for genetic programming.
FIG. 7.
Increase in UV fluorescence (450 to 460 nm) for sporangia, other fungal spores, and Poa pollen analyzed in the PAS-III after staining with increasing concentrations of Calcofluor white. MCN, median channel number (considered to be more reliable than the PCN for asymmetric or irregular distributions which were often found for fluorescence intensity). Separate datum points indicate the median channel number for samples analyzed on different days.
TABLE 2.
Effectiveness of Calcofluor white M2R for differentiation of P. infestans sporangia from other fungal spores and pollen grainsa
| Source | % Particles falling within gatesb defined for P. infestans sporangia when stained with Calcofluor white M2R at:
|
|||
|---|---|---|---|---|
| 0 μM | 10μM | 100 μM | 1 mM | |
| Alternaria sp. | 0 | 0 | 0 | 0 |
| Blumeria sp. | 5, 47, 65 | 0, 0, 0 | 0, 1, 2 | 0 |
| Brown rust | 0, 0 | 0 | 0, 0 | NT |
| Crown rust | 0, 2 | 0 | 0, 0 | NT |
| Poa annua pollen | 0, 0, 1 | 0, 0, 3 | 0, 0, 1 | 0, 4, 5 |
| P. infestans sporangia | 62, 67, 78, 87, 90, 92, 92, 93 | 69, 71, 77, 89, 89, 91, 92 | 77, 79, 81, 84, 85, 85, 91 | 36, 55, 67, 72 |
Data were analyzed by using multiple gating based on four parameters measured in the PAS-III: FSC, UV, and green and orange fluorescence. Figures separated by commas indicate experiments conducted on different days. NT, not tested.
The logical gating used was R1 and R2, where R1 and R2 were polygonal regions drawn from two-dimensional density plots of FSC versus green fluorescence and UV versus orange fluorescence, respectively, derived from data for sporangial suspensions. In the case of the other spores and pollens, data were first gated by using polygonal regions drawn from FSC versus green fluorescence plots for the individual data sets, in order to remove background particles.
Genetic programming proved to be an even more effective means of particle discrimination and allowed detection of 95% of the true positives with a false-positive rate of <1% on the holdout set. We note that the “ top” rule that was evolved contained a variety of nonlinear operators and that this type of approach is in contrast to the usual two- or three-dimensional displays where the separation of individual particles into classes via visual or computational clustering methods relies on them being linearly separable. This top rule can be cast as a small computer program as follows:
 |
 |
 |
 |
 |
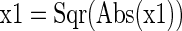 |
 |
 |
 |
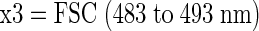 |
 |
 |
 |
If x1 ≠ 0, then x1 = x2/x1, else x1 = 1
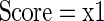 |
 |
A program of this nature, having inputs and outputs, can also be cast as an equation:
 |
if  ≠ 0
≠ 0
 |
where:
 |
 |
 |
 |
 |
 |
DISCUSSION
Different results were obtained for the FSC of biological particles when analyzed in the Microcyte compared with the PAS-III. This is to be expected since the precise range of angles at which“forward” scatter is collected differs between different instruments. In addition, the FSC in the Microcyte was recorded from the red diode laser (635 nm), whereas that of the PAS-III was recorded from the blue 488-nm laser. In both instruments, however, a more or less linear relationship between log (particle size) and FSC (also logarithmic) was observed for the “standard” beads. This is in agreement with other experiments with either beads or physiologically similar cells (e.g., references 11 and 38). However, a lower degree of correlation between particle size and FSC was observed for biological particles analyzed in the Microcyte, and in the Partec PAS-III (where a smaller range of sizes of particles was used) there was no correlation between size and FSC.
In addition to being influenced by particle size, the FSC is also influenced by the refractive index (relative to the surrounding medium), by the presence in cells, or on their surface, of compounds that may absorb light at the illumination wavelength used, or by highly textured surface or internal structures which may also act to decrease the intensity of FSC (37). These latter features have a greater influence on SSC than FSC, with SSC being less dependent upon particle size; typically, the highest-intensity SSC signals are obtained from particles with the highest degree of cytoplasmic granularity (12). Thus, in the present study, the greatly increased SSC and decreased FSC of killed sporangia compared with living sporangia (analyzed in the PAS-III) could be attributed in the former case to increased cytoplasmic granularity and in the latter case to damage to the cell membranes. Such damage would allow the surrounding medium to permeate the cells, reducing the difference in refractive index between the cell contents and the medium and so reducing the FSC (35).
It is perhaps fruitless to attempt to explain in detail the differences in FSC and SSC of the other biological particles, since their complex structures cannot be dealt with analytically by standard light-scattering theories. Clearly, the beads showed greater FSC, for their size, than most of the biological particles; this could be readily attributed to their higher refractive index (11). Possibly the relatively high FSC and SSC of sporangia, pollen grains, and Blumeria conidia might be related to the fact that they each possessed relatively unpigmented walls; perhaps the pigmentation in the walls of the other fungal conidia and rust spores served to absorb some of the incident light. For this study, the relevance of the observed differences in FSC and SSC lies not in determining their cause but rather in their usefulness for differentiating different spores and pollen types. This was possible to a limited extent among the particles tested, but sporangia could not be differentiated from Blumeria conidia by this method. As well as the (fairly small) differences in FSC and SSC, differentiation of pollen and urediniospores from sporangia was facilitated by the intrinsic orange and red fluorescence of the former. By using a combination of FSC, SSC, and fluorescence parameters, measured in the PAS-III, sporangia could be distinguished from other fungal spores (except Blumeria) and from the tested pollen types with fewer than 6% false positives. This was possible by using the multiple gating technique, which relies upon the ability to present data from up to six parameters as dot plots. This was not possible with data from the Microcyte, which recorded only two parameters, with data presented as histograms rather than as list-mode files. The Microcyte was effective at detecting populations of particles, but the PAS-III allowed individual particles to be assigned as false or true positives. Despite the advantages of portability offered by the Microcyte, it was concluded that only the PAS-III was sensitive enough to be useful for the aims of this present study, where small numbers of P. infestans sporangia must be detected against the varied background of the air spora.
The ability of fluorescent dyes to enhance particle differentiation, in particular, to allow differentiation of P. infestans sporangia from Blumeria conidia, was assessed for a variety of different “ red” dyes. Blumeria conidia are similar in size and shape to blight sporangia, although narrower and of more consistent dimensions and, like the sporangia, have a relatively unpigmented wall. Even so, it was thought likely that fluorescent dyes would stain these two spore types differently, since Blumeria is a true fungus (kingdom Fungi), whereas P. infestans is an alga-like oomycete or“pseudofungus” classified in the kingdom Chromista (9). As ascomycete fungi, Blumeria, Penicillium, Botrytis, and Alternaria spp. all have chitin and glucan as spore wall polymers, while the walls of Phytophthora spp. sporangia are composed of cellulose andβ -1,3-β-1,6-glucan (3, 4). However, for all the red dyes tested, it appeared that the thickness or pigmentation of the wall influenced the effectiveness of staining more than did the wall composition. Thus, the heavily pigmented conidia of Alternaria and Penicillium spp. were extremely resistant to all of the dyes tested. The less-pigmented conidia of Botrytis were slightly less resistant but still required more dye than did P. infestans sporangia or zoospores. The pattern of staining for Blumeria and pollen was similar to that for P. infestans sporangia. Moreover, the red dyes tended to mask differences in intrinsic fluorescence and so tended to make differentiation of sporangia from pollen and Blumeria less effective than could be achieved for unstained particles.
Calcofluor white M2R, one of a family of dyes known as fluorescent brighteners, proved more effective at differentiating sporangia from any of the other particles tested and had the additional advantage of being nontoxic and relatively inexpensive (13). Fluorescent brighteners have already been used in flow cytometric analyses of both fungi and bacteria (13, 23) and have been used to visualize sporangia of Phytophthora cactorum in the soil (19). In the present study, sporangia stained with Calcofluor white could be readily differentiated by using multiple gating from any of the other fungal spores tested, including Blumeria, with <1% false positives. Differentiation of sporangia from pollen grains with Calcofluor was more difficult since pollen stained in a similar manner to the sporangia but, even so, the use of genetic programming allowed differentiation of sporangia from pollen or from any of the other particles tested, again with <1% false positives at the level of the individual particle. We have used the same techniques successfully (staining with 10 μM Calcofluor white) to differentiate P. infestans sporangia from other pollen types including birch, sycamore, sweet vernal grass (Anthoxanthum odoratum), cocksfoot (Dactylis glomerata), and rough meadow grass (Poa trivialis), also with <1% false positives in each case (unpublished data). The usefulness of Calcofluor white stemmed from two factors. First, it effectively stained sporangia but did not stain the other fungal spores with the same intensity. Second, in contrast to the red stains, it did not mask the orange or red autofluorescence, which allowed the differentiation of sporangia from pollen. In addition, the nontoxic, stable nature of Calcofluor white makes it ideal for a system that is intended for use in field conditions.
In theory, use of genetic programming with data from the PAS-III would enable detection of only a single sporangium in a supplied sample. In practice, however, the detection threshold would need to be set higher than this in order to eliminate false positives. For example, if the system were set to trigger a warning for blight at, say, five sporangia per cubic meter of air, this would mean in theory that there could be up to 500 pollen grains present per cubic meter without triggering a warning (assuming <1% false positives for pollen). Preliminary experiments with a high volume air sampler (XMX/2AL Aerosol Concentrator/Liquid Sample Collector; Dycor Technologies, Ltd., Edmonton, Alberta, Canada) in infected potato fields at an ADAS trial site at Llanilar (Ceredigion, Wales, United Kingdom) in August and September 2000 (unpublished data) indicated that pollen and P. infestans sporangia were found at similar concentrations (ca. 19 to 30 per m3 of air). Alternaria spp. conidia were detected at much lower frequency (<2 per m3 of air); identification of conidia of Botrytis, Blumeria, and Penicillium spp. was not attempted.
Published studies (22) suggest that the mean number of pollen grains over an arable field may be as high as 600 per m3 in June, falling to in the order of 100 per m3 in July and fewer than 50 per m3 in August and September. Furthermore, “Alternaria type” conidia may on occasion be as numerous as 1,000 spores per m3 of air (22). In the same study it was found that P. infestans sporangia reached a maximum in August of over 60 sporangia per m3 (measured in the midst of a potato crop) but, clearly, in order to predict the disease it would be necessary to detect much lower concentrations than this.
The abundance of pollen (especially grass pollen) in the air in the earliest months of the “blight season” (June to July, or even May in blight-favorable years) will pose a major challenge for the detection of P. infestans sporangia by the methods described here. The first sporangia of the season must be detected against a background population of pollen that is likely to be greater by 2 orders of magnitude. The system evolved so far could be made to include further rules from an overall analysis of the patterns of particles so that it would not attach significance to 1% false positives. In addition, we will need to optimize the threshold at which a blight warning would be triggered, balancing the conflicting factors of the sensitivity of detection and the robustness of the system with respect to avoiding false positives. Consequently, we are currently developing the system further and are conducting trials for detection of P. infestans sporangia in field conditions against the naturally occurring background.
Acknowledgments
This work was supported by the Ministry of Agriculture, Fisheries, and Food, Horticulture and Potatoes Division (HP0132).
We thank Katherine Law, Hazel Davey, Dave Broadhurst, Helen Johnson, Leighton Pritchard, Mike Holland, Richard Lloyd, Richard Birch, David Summers, Pat Causton, Peter Henley, and John Parry of UW Aberystwyth; Tim Carver, Elwyn Jones, and Ian Thomas of the IGER; Nick Bradshaw and Aldwyn Clarke (ADAS); Bob Todd (Aber Instruments); Dale Travis and others at Dycor; and Carsten Herrmann, Volker Ost, and others at Partec GmbH.
REFERENCES
- 1.Allman, R. 1992. Characterization of fungal spores using flow cytometry. Mycol. Res. 96:1016–1018. [Google Scholar]
- 2.Banzhaf, W. P., R. E. Nordin, Keller, and F. D. Francone. 1998. Genetic programming: An introduction. Morgan Kaufmann, San Francisco, Calif.
- 3.Bartnicki-Garcia, S. 1968. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu. Rev. Microbiol. 22:87–108. [DOI] [PubMed] [Google Scholar]
- 4.Bartnicki-Garcia, S., and M. C. Wang. 1987. Biochemical aspects of morphogenesis in Phytophthora, p.121–137. In D. C. Erwin, S. Bartnicki-Garcia, and P. H. Tsao (ed.), Phytophthora: its biology, taxonomy, ecology, and pathology. American Phytopathological Society, St. Paul, Minn.
- 5.Bouma, E., and J. G. Hansen. 1999. Overview of standard descriptions of Phytophthora decision support systems, p.48–51. In H. Schepers and E. Bouma (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Uppsala, Sweden.
- 6.Bugiani, R., P. Govoni, and L. Cobelli. 1998. First large scale application of IPI model for potato late blight prediction in the Po valley, p.188–199. In H. Schepers and E. Bouma (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Carlow, Ireland.
- 7.Bugiani, R., P. Govoni, and L. Cobelli. 2000. Field evaluation of the combined use of IPI and different forecasting criteria for potato late blight control, p.266–275. In H. Schepers (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Oostende, Belgium.
- 8.Caten, C. E., and J. L. Jinks. 1968. Spontaneous variability of single isolates of Phytophthora infestans. I. Cultural variation. Can. J. Bot. 49:329–348. [Google Scholar]
- 9.Cavalier-Smith, T. 1998. A revised six-kingdom system of life. Biol. Rev. 73:203–266. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, A. 1993. Analysis of microalgae and cyanobacteria by flow cytometry, p.131–142. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, England.
- 11.Davey, H. M., C. L. Davey, and D. B. Kell. 1993. On the determination of the size of microbial cells using flow cytometry, p.49–65. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, England.
- 12.Davey, H. M., and D. B. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol. Rev. 60:641–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey, H. M., and D. B. Kell. 1997. Fluorescent brighteners: novel stains for the flow cytometric analysis of microorganisms. Cytometry 28:311–315. [DOI] [PubMed] [Google Scholar]
- 14.De Visser, C. L. M., and R. Meier. 2000. Field evaluation of four decision support systems for potato late blight in The Netherlands, p.137–155. In H. Schepers (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Oostende, Belgium.
- 15.Drenth, A., I. C. Q. Tas, and F. Govers. 1994. DNA fingerprinting uncovers a new sexually reproducing population of Phytophthora infestans in the Netherlands. Eur. J. Plant Pathol. 100:97–107. [Google Scholar]
- 16.Galbraith, D. W. 1989. Analysis of higher plants by flow cytometry and cell sorting. Int. Rev. Cytol. 116:165–228. [Google Scholar]
- 17.Gilbert, R. J., R. Goodacre, A. M. Woodward, and D. B. Kell. 1997. Genetic programming: a novel method for the quantitative analysis of pyrolysis mass spectral data. Anal. Chem. 69:4381–4389. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert, R. J., J. J. Rowland, and D. B. Kell. 2000. Genomic computing: explanatory modelling for functional genomics, p. 551–557. In D. Whitley, D. Goldberg, E. Cantú-Paz, L. Spector, I. Parmee, and H.-G. Beyer (ed.), Proceedings of the Genetic and Evolutionary Computation Conference (GECCO-2000). Morgan Kaufmann, Las Vegas, Nev.
- 19.Gisi, U. 1975. A new method for quantitative direct observation of sporangia of Phytophthora cactorum (Leb.et Cohn) Schroet. in the soil. Z. Pflanzenk. Pflanzen. 82:30–47. [Google Scholar]
- 20.Gjelsnes, O., and R. Tangen. 1994. Liquid flow cytometer. Norwegian patent WO 94/29695.
- 21.Goodacre, R., and R. J. Gilbert. 1999. The detection of caffeine in a variety of beverages using Curie-point pyrolysis mass spectrometry and genetic programming. Analyst 124:1069–1074. [Google Scholar]
- 22.Gregory, P. H., and J. M. Hirst. 1957. The summer air-spora at Rothamsted in 1952. J. Gen. Microbiol. 17:135–152. [DOI] [PubMed] [Google Scholar]
- 23.Hejtmánek, M., J. Dolezel, and I. Holubová. 1990. Staining of fungal cell walls with fluorescent brighteners: flow cytometric analysis. Folia Microbiol. 35:437–442. [DOI] [PubMed] [Google Scholar]
- 24.Hijmans, R. J., G. A. Forbes, and T. S. Walker. 2000. Estimating the global severity of potato late blight with GIS-linked disease forecast models. Plant Pathol. 49:697–705. [Google Scholar]
- 25.Hinds, H. 2000. Using disease forecasting to reduce fungicide input for potato blight in the UK, p.83–90. In H. Schepers (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Oostende, Belgium.
- 26.Johnson, H. E., R. J. Gilbert, M. K. Winson, R. Goodacre, A. R. Smith, J. J. Rowland, M. A. Hall, and D. B. Kell. 2000. Explanatory analysis of the metabolome using genetic programming of simple, interpretable rules. Genet. Prog. Evolvable Machines 1:243–258. [Google Scholar]
- 27.Kell, D. B., and B. Sonnleitner. 1995. GMP—Good Modelling Practice: an essential component of good manufacturing practice. Trends Biotechnol. 13:481–492. [Google Scholar]
- 28.Kleinhenz, B., and E. Jörg. 2000. Results of validation trials of Phytophthora DSS in Europe in 1999, p.180–190. In H. Schepers (ed.), Proceedings of the Workshop on the European Network for Development of an Integrated Control Strategy of Potato Late Blight. PAV, Oostende, Belgium.
- 29.Koza, J. R. 1992. Genetic programming II: on the programming of computers by means of natural selection. MIT Press, Cambridge, Mass.
- 30.Koza, J. R. 1994. Genetic programming II: Automatic discovery of reusable programs. MIT Press, Cambridge, Mass.
- 31.Koza, J. R., F. H. Bennett, M. A. Keane, and D. Andre. 1999. Genetic programming III: Darwinian invention and problem solving. Morgan Kaufmann, San Francisco, Calif.
- 32.Langdon, W. B. 1998. Genetic programming and data structures: genetic programming + data structures = automatic programming! Kluwer, Boston, Mass.
- 33.Muirhead, K. A., P. K. Horan, and G. Poste. 1985. Flow cytometry: present and future. Bio-technology 3:337–356. [Google Scholar]
- 34.Porter, J. D., D. Deere, M. Hardman, C. Edwards, and R. Pickup. 1997. Go with the flow—use of flow cytometry in environmental microbiology. FEMS Microbiol. Ecol. 24:93–101. [Google Scholar]
- 35.Salzman, G. C., S. B. Singham, R. C. Johnston, and C. F. Bohren. 1990. Light scattering and cytometry, p.81–107. In M. R. Melamed, T. Lindmo, and M. L. Mendelsohn (ed.), Flow cytometry and sorting, 2nd ed. Wiley-Liss, New York, N.Y.
- 36.Schlenzig, A., J. Habermeyer, and V. Zinkernagel. 1998. Monitoring of epidemic development of Phytophthora infestans in potato crops on the basis of sporangia movement. Z. Pflanzenk. Pflanzen. 105:22–33. [Google Scholar]
- 37.Shapiro, H. M. 1995. Practical flow cytometry, 3rd ed., p.1–31; 231–232. Alan R. Liss, Inc., New York, N.Y.
- 38.Sharpless, T. K., M. Bartholdi, and M. R. Melamed. 1977. Size and refractive index dependence of simple forward angle scattering measurements in a flow system using sharply focused illumination. J. Histochem. Cytochem. 25:845–856. [DOI] [PubMed] [Google Scholar]
- 39.Sujkowski, L. S., S. B. Goodwin, A. T. Dyer, and W. E. Fry. 1994. Increased genotypic diversity via migration and possible occurrence of sexual reproduction of Phytophthora infestans in Poland. Phytopathology 84:201–207. [Google Scholar]
- 40.Tarran, G. A., and P. H. Burkill. 1993. Flow cytometry at sea, p.143–158. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, England.
- 41.Taylor, J., R. Goodacre, W. G. Wade, J. J. Rowland, and D. B. Kell. 1998. The deconvolution of pyrolysis mass spectra using genetic programming: application to the identification of some Eubacterium species. FEMS Microbiol. Lett. 160:237–246. [DOI] [PubMed] [Google Scholar]
- 42.Vives-Rego, T., P. Lebaron, and G. Nebe-von-Caron. 2000. Current and future applications of flow cytometry in aquatic microbiology. FEMS Microbiol. Rev. 24:429–448. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins, M. F., L. Boddy, C. W. Morris, and R. R. Jonker. 1999. Identification of phytoplankton from flow cytometry data by using radial basis function neural networks. Appl. Environ. Microbiol. 65:4404–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winson, M. K., and H. M. Davey. 2000. Flow cytometric analyses of microorganisms. Methods 21:231–240. [DOI] [PubMed] [Google Scholar]
- 45.Woodward, A. M., R. J. Gilbert, and D. B. Kell. 1999. Genetic programming as an analytical tool for non-linear dielectric spectroscopy. Bioelectrochem. Bioenerg. 48:389–396. [DOI] [PubMed] [Google Scholar]