Abstract
The population dynamics of pseudomonads in gilt-head sea bream Mediterranean fish (Sparus aurata) stored under different conditions were studied. Phenotypic analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins were performed to identify a total of 106 Pseudomonas strains isolated from S. aurata stored under different temperatures (at 0, 10, and 20°C) and packaging conditions (air and a modified atmosphere of 40% CO2-30% N2-30% O2). Pseudomonas lundensis was the predominant species, followed by Pseudomonas fluorescens, while Pseudomonas fragi and Pseudomonas putida were detected less frequently. Fluorescent Pseudomonas strains dominated under air conditions, while proteolytic and less lipolytic strains dominated under modified-atmosphere packaging. Different storage conditions appear to govern the selection of pseudomonads in gilt-head sea bream fish.
It is well known that fish spoilage is primarily due to (i) autolysis, (ii) bacterial growth and metabolism resulting in the formation of off-flavor compounds, and (iii) chemical oxidation of lipids. Among these reasons, microbiological activity is by far the most important factor influencing fish quality (22). However, not all microorganisms in seafood are equally important for quality changes. Fish feeding habits, geographical location, season, sea temperature, type of fish, place in which the fish were harvested, and storage conditions, including temperature and composition of the packaging atmosphere, determine the spoilage domains of specific spoilage organisms (SSO) (22, 36).
Photobacterium phosphoreum, Shewanella putrefaciens, Brochothrix thermosphacta,Pseudomonas spp., Aeromonas spp., and lactic acid bacteria were found to be members of the microbial association in fish from temperate waters (12, 32). However, among these, only S. putrefaciens was the SSO of marine cold-water fish stored in ice, while P. phosphoreum was the SSO of fish stored under modified-atmosphere conditions. On the other hand, Pseudomonas spp. and Shewanella spp. were found to be the SSO in fish obtained from Mediterranean Sea temperate waters and stored in ice aerobically (32). In comparison to data for meat and meat products (19, 45, 57), identification and characterization of SSO in fish under different storage conditions have not been sufficiently studied. This situation is especially evident with Pseudomonas spp., a group with high heterogeneity and biodiversity within species and/or subspecies. Furthermore, many pseudomonad groups have no clear taxonomic status or natural relationships with other genera (44, 47, 48, 49).
For both of the reasons mentioned above, the use of conventional phenotypic methods can offer only limited results (6, 9, 42). In contrast, molecular methods are powerful tools not only for identification at the species level but also for strain characterization (7, 14, 29, 30, 63). Although molecular fingerprinting methods have been successfully applied for bacteria of medical interest in epidemiological studies, they are not always suitable for tracking a particular strain in the food environment (10). The use of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), an advanced phenotypic method which falls between conventional phenotypic and molecular methods, could be of great importance. Indeed, the electrophoretic separation of cellular proteins is a sensitive technique, providing information on the similarity of strains at the (sub)species level (64).
Therefore, the aims of the present study were (i) to elucidate the influence of storage conditions, such as temperature and packaging atmosphere, on the selection of these bacteria and (ii) to understand the diversity of pseudomonas populations on fish by characterizing isolated Pseudomonas strains by using both conventional phenotypic analysis and SDS-PAGE of whole-cell proteins.
MATERIALS AND METHODS
Preparation of fish.
Fresh, gutted gilt-head sea bream (Sparus aurata) was stored in ice immediately after capture. The fish were kept in ice in a local fishery shop until they were bought within 6 to 8 h after capture and were transported in ice within 30 to 45 min from their purchase to the laboratory. On arrival at the laboratory, they were divided into six portions, which were kept at 0, 10, and 20°C. The fish were stored in individual pouches aerobically and under modified-atmosphere packaging (MAP) conditions (40% CO2-30% N2-30% O2) in a Suprovac polyamide laminate packaging membrane (thickness, 90 μm; gas permeability at 20°C and 50% rH, ca. 25, 90, and 6 cm3 m−2 day−1 bar−1 [1 bar= 105 Pa] for CO2, O2, and N2, respectively). Samples were taken at appropriate time intervals to monitor microbial growth.
Sample preparation and microbiological analysis.
A 25-g portion from the dorsal half of the fish was transferred to a stomacher bag (Seward Medical, London, United Kingdom); 225 ml of 0.1% peptone water with salt (NaCl, 0.85% [wt/vol]) was added, and the suspension was homogenized for 60 s with a stomacher (Lab Blender 400; Seward Medical). Samples (0.1 ml) of serial dilutions of fish homogenates were spread on the surfaces of appropriate media in petri dishes. (i) For the enumeration of pseudomonads, cetrimide-fusidin-cephaloridine agar (code CM 559, supplemented with SR 103; Oxoid, Basingstoke, United Kingdom) was incubated at 20°C for 2 days (40). (ii) Lactic acid bacteria and B. thermosphacta were also counted by use of MRS and STAA media (Oxoid), respectively (32). Three replicates of at least three appropriate dilutions were enumerated. All plates were examined visually for typical colony types and morphological characteristics associated with the growth medium.
Curve fitting.
The growth data from the plate counts were transformed to log10 values. The Baranyi model (4) was fitted to the logarithm of the viable cell concentration. For curve fitting, the in-house program DMFit (Institute of Food Research, Reading, United Kingdom) was used; it was kindly provided by J. Baranyi.
Isolation and purification of strains.
All plates with countable colonies were examined for typical colony types and morphological characteristics. Colonies were selected from petri dishes having an average of 30 to 50 colonies. Colonies were purified by streaking on inoculated nutrient agar at 4°C for 3 to 5 days. Strains were examined for physiological characteristics such as the following: Gram staining (24), cell morphology, flagellar arrangement (39), oxidase reaction (33), catalase formation, aerobic and anaerobic breakdown of glucose (27), ammonia production from arginine (61), acid production from maltose (11), decarboxylation of ornithine (18), lipolytic activity (55), production of fluorescent pigment (31), and growth at different temperatures (41).
Reference strains F 324 (Pseudomonas fluorescens biovar V-1), F 384 (P. fluorescens biovar V-5), F 335 (P. fluorescens biovar III-1), F 388 (P. fluorescens biovar I-1), F 10553 (P. fragi), F 385 (P.putida biotype A), F 214 (P.lundensis), F 46 (P. putida), and F 47 (P. fragi) isolated from spoiled fish and fresh fish were kindly provided by Mario Gennari (Instituto Ispezione Degli Alimenti Di Origine Animale, Milan, Italy) and Paw Dalgaard (Danish Ministry of Fisheries, Institute of Fish Research, Lyngby, Denmark), respectively.
Assimilation of carbon sources.
The basal medium described by Molin and colleagues (41, 42) and containing 0.1% (wt/vol) filter-sterilized carbon sources was used. The following carbon sources were tested: d-arabinose, arabitol, dl-carnitine, creatine, deoxycholate, d-galactonate, d-glucuronate, 4-hydroxy-benzoate, hydroxy-l-proline, inosine,meso-inositol, malonate, d-mannitol, mucate, d-quinate,d-saccharate, d-xylose, andd-glucose. Strains were grown overnight on nutrient agar at 25°C. Then, a colony was transferred to 10 ml of nutrient broth and incubated for 18 h at 25°C. Cells were collected aseptically by centrifugation at 4°C for 15 min at 13,518 × g, washed twice with physiological saline (0.85% [wt/vol] NaCl), and finally resuspended in 1.0 ml of physiological saline. Washed cells (>106 cells per ml) were plated on microplates containing media and incubated at 25°C. Growth was assessed on days 1, 4, 7, 12, and 14 by using a microtiter plate reader. Tests were performed in triplicate. A blank without an added carbon source and a standard with added glucose were also used as described by Drosinos (15).
Preparation of cell extracts.
Strains were subcultured in 9 ml of nutrient broth for 24 h at 25°C. They were then grown on nutrient agar for 48 h at 25°C. Cells were collected and washed with phosphate buffer (pH 7.3; 10 mmol liter−1) containing 0.8% NaCl. Cell extracts were prepared from approximately 100 mg of bacterial cells (wet weight) and suspended in 1 ml of Tris-HCl buffer (pH 6.8; 62 mmol liter−1) containing 2% sodium dodecyl sulfate, 5% (vol/vol) mercaptoethanol, and 10% (wt/vol) glycerol. The cell suspension was heated for 10 min at 100°C, cooled on ice, and centrifuged at 4°C for 15 min at 10,000 rpm. The supernatant obtained (protein extract) was stored at−20°C and used for SDS-PAGE analysis (63).
PAGE of proteins.
Whole-cell protein extracts were prepared as described above. Registration of the protein electrophoretic patterns, normalization of the densitometric traces, grouping of strains by the Pearson product-moment correlation coefficient, and Unweighted Pair Group Method Using Arithmetic Averages (UPGMA) cluster analysis were performed as described by Pot et al. (51) using the software package GelCompar (version 4.0; Applied Maths, Kortrijk, Belgium).
RESULTS
Changes in the population of pseudomonads.
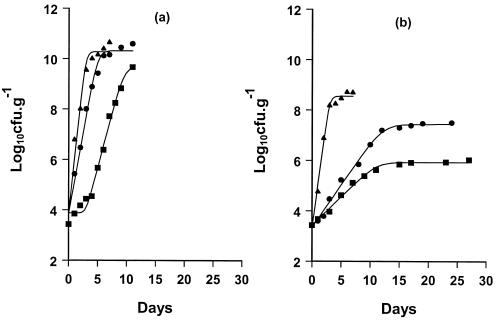
The growth of Pseudomonas in fish stored under different packaging conditions is shown in Fig. 1. The growth rate for pseudomonads under both packaging conditions (Fig. 1) was higher at 20°C than at the other two temperatures (10 and 0°C). Pseudomonads were the dominant organisms under aerobic conditions, while lactic acid bacteria and B. thermosphacta were the dominant microbial associations in gilt-head fish stored in a modified atmosphere (results not shown).
FIG. 1.
Changes in the Pseudomonas population of naturally spoiled gilt-head sea bream fish during storage in air (a) and with MAP (b) at 0°C (▪), 10°C (•), and 20°C (▴), as fitted with the Baranyi model.
Characterization of Pseudomonas spp.
From a total of 150 isolated and purified colonies, 106 were further subjected to physiological tests and to SDS-PAGE analysis of whole-cell proteins, as described in Materials and Methods. All of the strains were gram negative, were catalase and oxidase positive, showed oxidative metabolism on Hugh-Leifson medium, could hydrolyze arginine, and could grow at 4°C. None of them decarboxylated ornithine, produced phenazine pigment, or grew at 42°C. All of the strains were able to assimilate arabitol, hydroxy-l-proline, d-mannitol, d-quinate, and d-glucose. On the contrary, creatine and mucate were not assimilated. Sixty-five percent of the strains produced fluorescent pigment on King medium B, while proteolytic and lipolytic activities as well as acid production from maltose and assimilation of 11 further carbon sources varied among the strains (Table 1).
TABLE 1.
Phenotypic characteristics that differentiate groups of Pseudomonas strains
| Characteristic | Reactiona of the following Pseudomonas strains (n):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cluster
|
Reference
|
||||||||
| I (17) | II (36) | III (4) | IV (45) | V (4) | P. fragi (cluster II) (2) | P. putida(cluster II) (2) | P. fluorescens (cluster II) (4) | P. lundensis (cluster IV) (1) | |
| Oxidase reaction | + | + | + | + | + | + | + | + | + |
| Catalase reaction | + | + | + | + | + | + | + | + | + |
| Oxidative metabolism on Hugh & Leifson medium | + | + | + | + | + | + | + | + | + |
| Ammonia from arginine | + | + | + | + | + | + | + | + | + |
| Growth at 4°C | + | + | + | + | + | + | + | + | + |
| Growth at 42°C | − | − | − | − | − | − | − | − | − |
| Production of phenazine | − | − | − | − | − | − | − | − | − |
| Ornithine decarboxylase | − | − | − | − | − | − | − | − | − |
| Production of lipase | − | − | + | − | − | − | − | d | − |
| Production of protease | − | + | + | d | + | + | − | + | + |
| Production of fluorescent pigment | d | + | + | + | d | − | + | + | + |
| Acid production from maltose | − | − | − | + | + | + | − | − | + |
| Assimilation of carbon sources: | |||||||||
| Creatine | − | − | − | − | − | + | − | d | − |
| Mucate | − | − | − | − | − | − | − | − | − |
| Arabitol | + | + | + | + | + | + | + | + | + |
| Hydroxy-l-proline | + | + | + | + | + | + | + | + | + |
| d-Mannitol | + | + | + | + | + | + | + | + | + |
| d-Glucose | + | + | + | + | + | + | + | + | + |
| d-Quinate | + | + | + | + | + | + | + | + | + |
| d-Xylose | d | + | + | + | + | + | + | + | + |
| l-Arabinose | d | d | + | + | d | + | − | − | + |
| dl-Carnitine | d | + | + | − | − | + | + | + | − |
| Deoxycholate | d | d | − | + | d | + | + | + | + |
| d-Galactonate | d | + | − | − | − | + | + | + | − |
| d-Glucuronate | d | d | d | − | d | + | + | + | − |
| 4-Hydroxy-benzoate | d | d | − | − | d | + | + | + | − |
| Inosine | d | d | − | − | − | + | + | + | − |
| meso-Inositol | d | + | d | + | d | + | + | + | + |
| Malonate | d | d | − | − | − | + | + | + | − |
| d-Saccharate | + | d | − | − | d | + | + | + | − |
+, 65% or more the total; −, 35% or less the total; d, 36 to 64% the total.
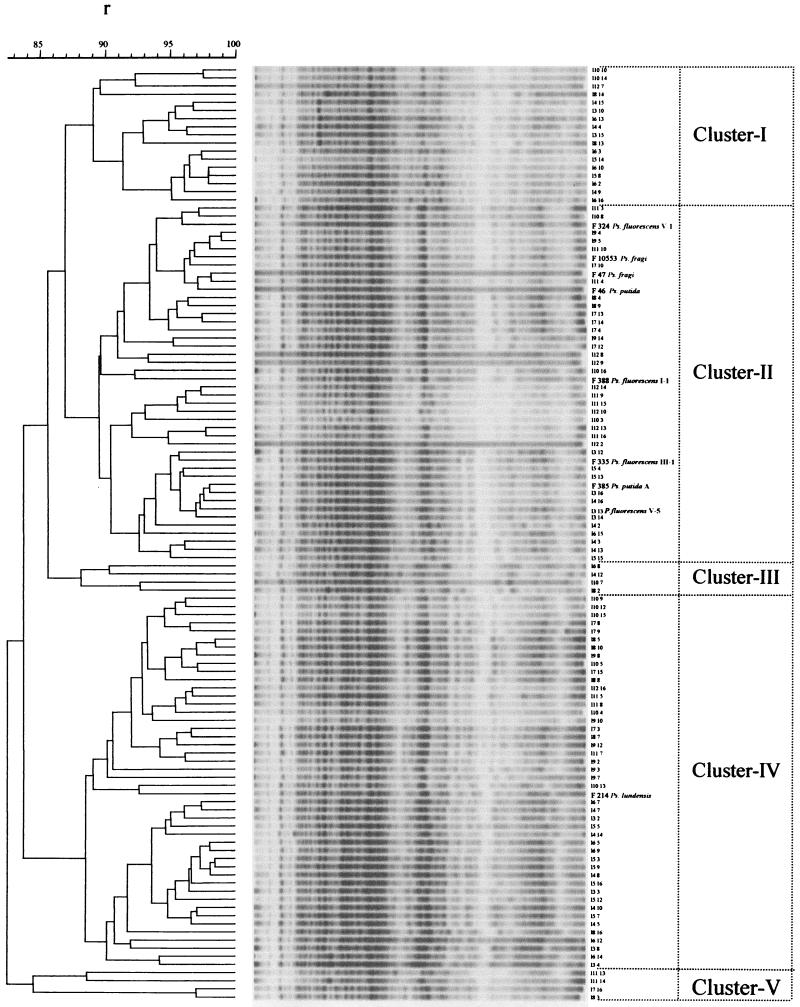
The computerized numerical analysis of the protein electropherograms revealed five main clusters. The reproducibility of the SDS-PAGE technique was estimated by including duplicate runs of a single protein extract on separate gels (r, ≥0.94). A good correlation between the clusters derived by the SDS-PAGE analysis (Fig. 2) and the phenotypic characterization (Table 1) was noted and is discussed below.
FIG. 2.
Clustering of protein electropherograms of 115 strains of Pseudomonas. The Pearson product-moment correlation coefficient and the UPGMA method were used. The horizontal scale represents percent similarities. The vertical scale recognizes the strains and clusters of Pseudomonas strains.
Cluster I included 17 strains with a similarity level of ≥0.89. Only 5 and 2 out of the 17 strains were proteolytic and lipolytic, respectively. Fluorescent pigment was produced by 47% of these strains, and most of them could assimilate the majority of the carbon sources tested. Concerning origin (Table 2), the main portion of the strains (64.7%) was derived from samples stored at 10°C under aerobic conditions, followed by strains isolated from fish stored at 20°C, again under aerobic conditions (17.7%). Finally, two strains belonged to the initial fish microflora, while one strain was isolated from fish stored under MAP conditions at 20°C.
TABLE 2.
Clusters of Pseudomonas spp. and proportions of proteolytic and lipolytic strains isolated from gilt-head sea bream stored under aerobic and MAP conditions at 0, 10, and 20°C
| Cluster | No. (%) of strains in the indicated conditions:
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total
|
Proteolytic
|
Lipolytic
|
||||||||||||||||||||||
| Initial flora | Air
|
MAP
|
Total | Initial flora | Air
|
MAP
|
Total | Initial flora | Air
|
MAP
|
Total | |||||||||||||
| 0°C | 10°C | 20°C | 0°C | 10°C | 20°C | 0°C | 10°C | 20°C | 0°C | 10°C | 20°C | 0°C | 10°C | 20°C | 0°C | 10°C | 20°C | |||||||
| I | 2 (14.3) | 11 (37.9) | 3 (21.4) | 1 (25.0) | 17 | 1 (50.0) | 2 (18.2) | 2 (66.7) | 5 (29.4) | 1 (50.0) | 1 (9.09) | 2 (11.8) | ||||||||||||
| II | 3 (21.5) | 8 (23.6) | 10 (34.5) | 8 (57.2) | 5 (62.5) | 2 (66.7) | 36 | 2 (66.6) | 4 (50.0) | 7 (70.0) | 8 (100) | 5 (100) | 2 (100) | 28 (77.8) | 1 (33.3) | 6 (60.0) | 1 (20.0) | 8 (22.2) | ||||||
| III | 1 (7.10) | 3 (8.80) | 4 | 1 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 4 (100) | |||||||||||||||
| IV | 7 (50.0) | 23 (67.6) | 8 (27.6) | 3 (21.4) | 3 (37.5) | 1 (33.3) | 45 | 4 (57.1) | 9 (39.1) | 2 (25.0) | 1 (33.3) | 1 (33.3) | 1 (100) | 18 (40) | 4 (57.1) | 3 (13.0) | 1 (12.5) | 1 (33.3) | 1 (100) | 10 (22.2) | ||||
| V | 1 (7.10) | 3 (75.0) | 4 | 1 (100) | 3 (100) | 4 (100) | 1 (100) | 1 (25.0) | ||||||||||||||||
| Total | 14 (100) | 34 (100) | 29 (100) | 14 (100) | 8 (100) | 3 (100) | 4 (100) | 106 | 9 (64.3) | 16 (47.1) | 11 (37.9) | 11 (78.6) | 6 (75.0) | 3 (100) | 3 (75.0) | 59 (55.7) | 8 (57.1) | 6 (17.6) | 8 (27.6) | 2 (25.0) | 1 (33.3) | 25 (23.6) | ||
A total of 36 strains grouped together in cluster II at an r value of ≥0.895. All strains produced fluorescent pigment, 77.8% exhibited proteolytic activity, but only 22.2% were lipolytic. The majority of the strains were derived from samples stored under aerobic conditions (Table 2), mainly at 10°C (27.7%) and in a slightly lesser degree from fish stored at 0°C (22.2%) and 20°C (22.2%). However, seven strains were isolated from fish stored under MAP conditions, either at 0°C (13.9%) or at 10°C (5.6%). Finally, three strains belonged to the initial fish microflora. It is interesting that despite their distinct phenotypic profiles, eight reference strains belonging to three different Pseudomonas species, namely, F 324 (P.fluorescens biovar V-1), F 384 (P.fluorescens biovar V-5), F 335 (P.fluorescens biovar III-1), F 388 (P.fluorescens biovar I-1), F 10553 (P.fragi), F 47 (P. fragi), F 385 (P. putida biotype A), and F 46 (P.putida), grouped together with the 36 strains of cluster II.
Cluster III comprised four strains at an r value of ≥0.88; these were the only ones among all the strains that showed both proteolytic and lipolytic activities. All four strains produced fluorescent pigment and could assimilate a rather limited number of carbon sources. Three were derived from samples stored at 0°C under aerobic conditions, while one belonged to the endogenous initial fish microflora (Table 2).
Cluster IV comprised 45 strains at an r value of ≥0.88. Only 10 strains (22.2%) were lipolytic, while 40% of the strains showed proteolytic activity and 45% were able to produce fluorescent pigment. Only 25% of the 45 strains were both proteolytic and fluorescent. Strains in cluster IV exhibited the poorest carbon source assimilation among all of the strains examined in this study; however, they all produced acid from maltose. Almost half of the strains (51.1%) were derived from fish stored at 0°C under aerobic conditions (Table 2). Four strains (8.9%) originated from samples stored under MAP conditions, while seven strains (15.6%) belonged to the initial fish microflora. The identical phenotype of cluster IV and the reference strain F 214 (P. lundensis), which was also included in this cluster, suggested that these 45 strains are closely related to P. lundensis.
Finally, four strains grouped at an r value of ≥0.84 in cluster V. They were all proteolytic, producing at the same time acid from maltose. Fifty percent of these strains produced fluorescent pigment. Finally, they showed fairly good assimilation of carbon sources. Three strains were derived from samples stored at 20°C under MAP conditions, while only one belonged to the initial fish microflora (Table 2).
DISCUSSION
It is well known that pseudomonads are ubiquitous bacteria in nature. Due to their ability to utilize a wide range of organic compounds, they occupy an important ecological position in the carbon cycle. Therefore, the ecology of pseudomonads in the biosphere has been a matter of interest. In this sense, an accurate system for the classification and identification of pseudomonads is an essential prerequisite for a detailed investigation of the roles and evolution of these bacteria originating in either animal (e.g., fish and meat) or plant products.
However, the classification of Pseudomonas strains is problematic due to the lack of an accurate taxonomic system (67). The need for taxonomic revision of Pseudomonas spp. was emphasized in the early studies of Ayres (2). At that time, the classification, nomenclature, and identification of species of this genus, the trinity that Cowan et al. (11) referred to as taxonomy, were wholly unsatisfactory. This situation resulted in many poorly defined species of the genus in the reports of most investigators (58), especially those of food microbiologists (2). Different identification schemes based on multiple physiological characters, such as carbon source utilization and pigment formation, have been suggested and used for species differentiation, but with doubtful success (19). Advanced phenotypic methods, such as whole-cell protein fingerprinting, fatty acid profiling (mainly 2- and 3-hydroxy fatty acids), and enzyme-linked immunosorbent assaying with polyclonal antibodies against live cells, have proved to be more efficient and reliable for the differentiation and classification of pseudomonads (53, 59).
In recent years, molecular methods, such as DNA-rRNA hybridization (14, 50), DNA-DNA hybridization (62), direct comparison of rRNA sequences (37), comparison of macrorestriction patterns by pulsed-field gel electrophoresis (25), and gel electrophoresis of stable low-molecular-weight components of the rRNA pool (26), have been widely applied in order to establish the phylogenetic relationships between new isolates and previously defined taxa. However, no matter what methodology is used for the identification of Pseudomonas strains, there is always confusion.
In the present study, both the phenotypic characterization and the SDS-PAGE analysis grouped the pseudomonad isolates in five clusters (Table 1 and Fig. 2). Of the five clusters obtained, several (cluster I to IV) could be equated, on the basis of comparisons with published data (5, 42, 54), with recognized species at a high degree of certainty (Table 1).
Clustering of pseudomonads in five major groups was proposed in the classic studies by Stanier et al. (58). This classification of species appeared to have been formulated by comprehensive testing of phenotypic attributes of isolates (i.e., for their ability to use a wide range of organic substrates as sole carbon and energy sources for aerobic growth). Subsequently, it was shown that each group was a relatively closely circumscribed rRNA-DNA hybridization group (50). It has been shown, however (65), that only one of the groups, the fluorescent one of Stanier et al. (58), is Pseudomonas sensu strictu. All pseudomonads isolated from meat belong to this genus. Such information is lacking as far as the pseudomonads of fish isolates are concerned.
The approach of Stanier et al. (58) was applied to isolates from meat and meat products without success (13). In retrospect, this was not an unexpected result for the simple reason that Stanier et al. (58) did not include isolates from any foods, e.g., vegetable, meat, or other chilled proteinaceous foods (e.g., fish), in their culture collection. Subsequent studies by Banks and Board (3), Molin and Ternstrom (41), and Shaw and Latty (54) endorsed this observation, and various biovars of P. fragi became widely accepted as the principal aerobic gram-negative spoilage organisms in the microbial associations of meat and certain meat products at chill temperatures. This was not a new species sensu strictu. It had been isolated from meat and named by Eichholz (17), had been tentatively placed in the rRNA group I (47), and had been shown by Hussong et al. (28) to be an important spoilage organism in the dairy industry. Further studies by Molin and Ternstrom (42), Molin et al. (43), and Prieto et al. (52) of meat (beef or lamb) defined a new species,P. lundensis. P.fluorescens and the two species noted above constitute a significant part of the spoilage microflora on chilled meat stored in an aerobic atmosphere. In practice, members of the P.fragi complex and P. lundensis were the dominant organisms in the vast majority of the meat samples examined by these authors.
In the present study, with both physiological characteristics and whole-cell protein profiles, it was not possible to differentiate P. fluorescens from P.fragi and P. putida isolates, which grouped together in cluster II. These results confirm those of previous reports, where even DNA-RNA hybridization and 23S ribosomal DNA sequencing (5, 9, 14, 38) were not enough to distinguish properly these three species. However, other studies have not revealed a close relationship among these species (41, 42, 54). Only for P. fluorescens biovar V was it shown that this species was similar either to P. fragi (5, 38) or to P. putida (8). Indeed, in the present study, P.fluorescens biovar V was found to be close to the reference strains of P. fragi and P. putida on the basis of whole-cell protein profiles. Considering the close relationships among those three species, many authors have suggested the reclassification of the Pseudomonas genus (1, 56, 67). We designated cluster II strains as belonging to the P.fluorescens complex. Indeed, the physiological characteristics of these strains precluded the possibility that they were P. putida, whereas two strains had a phenotype similar to that of P. fragi (47) (Table 1).
The physiological characteristics of the strains in cluster IV, as well as the fact that the reference strain of P.lundensis clusters with them in the SDS-PAGE analysis of whole-cell proteins, suggest that these 45 strains belong to the species P. lundensis (43, 60). A phenotypic profile similar to that of P.lundensis was obtained for the strains in cluster V as well. However, the SDS-PAGE analysis could differentiate these strains from those in cluster IV.
The succession of pseudomonads during aerobic storage of meat and fish at low temperatures is well established (19, 21, 22, 32). It was evident that the taxonomic composition of the pseudomonad population was determined largely by the relative incidence of each taxon in the initial contamination. This finding may imply that the absence of change in proportions with storage is due to the taxa having similar growth rates. However, this notion is under dispute. The observation of Lebert et al. (35) that strains of P. fragi had shorter lag times than those of P. fluorescens partly explains the domination of the former over the latter during the chilling process in meat and fish, despite the higher initial incidence of P.fluorescens (21, 23, 34).
In fish samples, P. fragi and P.fluorescens were also the major members of the pseudomonads, but only a few isolates were characterized as P.lundensis (20, 60). In the present study, it was found that there was an important contribution of the P.lundensis group initially and at the end of storage of gilt-head sea bream fish at low temperatures. The population of P. fluorescens followed, while that of P. fragi was limited. The temperature of storage and the composition of the atmosphere affected the contributions of the above-mentioned groups in the final population of pseudomonads in fish (Table 2).
Indeed, it is well known that the contribution of pseudomonads to the final microbial association of fish or meat depends on the composition of the atmosphere as well as on the permeability of the film used for the packaging of these products (12, 57). In this study, it was evident that there was a further selection among the different pseudomonad groups as far as the storage conditions were concerned. Indeed, strains of clusters I and V were found mainly at higher storage temperatures (10 and 20°C) in both aerobic and MAP conditions. On the contrary, strains of clusters II and IV were able to grow under aerobic conditions at all tested temperatures and at chill temperatures (0 and 10°C) under MAP conditions.
The ability of Pseudomonas spp. to grow in refrigerated meat and fish is due partly to their distinct metabolism of glucose (46). Drosinos and Board (16) found metabolic differences among Pseudomonas spp. from meat. They proposed that the dominance of P. fragi over P.lundensis and P. fluorescens is due to its ability to metabolize creatine and creatinine under aerobic conditions but not under modified atmospheres. The facts that in fish the concentration of glucose is rather limited and that the selected strains were unable to assimilate creatine could partly explain the dominance of proteolytic strains (Tables 1 and 2) of the fluorescent group and the failure of P. fragi to become dominant in fish under either type of storage conditions. The interaction between proteolytic and nonproteolytic strains should also be taken into account (66).
The present study revealed that P. lundensis and P. fluorescens biovars are likely to be more common and that P. fragi and P.putida are likely to be less common in fish that originating from the Mediterranean Sea. More research is needed in this field.
Acknowledgments
This research was funded by the Greek Ministry of Development (GSRT-EKVAN project 21). One of us (P.T.) thanks the Greek Scholarship Foundation for financial support of her Ph.D. thesis.
REFERENCES
- 1.Anzai, Y., Y. Kudo, and H. Oyaizu. 1997. The phylogeny of the genera Chryseomonas, Flavimonas and Pseudomonas supports synonymy of those three genera. Int. J. Syst. Bacteriol. 47:249–251. [DOI] [PubMed] [Google Scholar]
- 2.Ayres, J. C. 1960. The relationship of organisms of the genus Pseudomonas to the spoilage of meat, poultry and eggs. J. Appl. Bacteriol. 23:471–486. [Google Scholar]
- 3.Banks, J. G., and R. G. Board. 1983. The classification of pseudomonads and other obligately aerobic Gram-negative bacteria from British pork sausage and ingredients. Syst. Appl. Microbiol. 4:424–438. [DOI] [PubMed] [Google Scholar]
- 4.Baranyi, J., T. A. Roberts, and P. McClure. 1993. A non-autonomous differential equation to model bacterial growth. Food Microbiol. 10:43–59. [Google Scholar]
- 5.Barrett, E., R. E. Solanes, J. Tang, and N. J. Palleroni. 1986. Pseudomonas fluorescens biovar V: its resolution into distinct component groups and the relationship of these groups to other P.fluorescens biovars, to P. putida and to psychrotrophic pseudomonads associated with food spoilage. J. Gen. Microbiol. 132:2709–2721. [DOI] [PubMed] [Google Scholar]
- 6.Baumann, P., L. Baumann, M. Mandel, and R. Allen. 1972. Taxonomy of aerobic marine eubacteria. J. Bacteriol. 110:402–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennasar, A., J. Guasp, and J. Lalucat. 1998. Molecular methods for the detection and identification of Pseudomonas stutzeri in pure culture and environmental samples. Microb. Ecol. 35:22–53. [DOI] [PubMed] [Google Scholar]
- 8.Champion, A. B., E. L. Barrett, N. J. Palleroni, K. L. Soderberg, R. Kunisawa, R. Contopoulou, A. C. Wilson, and M. Doudoroff. 1980. Evolution in Pseudomonas fluorescens. J. Gen. Microbiol. 120:485–511. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, H., M. Boye, L. K. Poulsen, and O. F. Rasmussen. 1994. Analysis of fluorescent pseudomonads based on 23S ribosomal DNA sequences. Appl. Environ. Microbiol. 60:2196–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costas, M., H. B. Olmes, S. L. W. On, and D. E. Stead. 1992. Identification of medically important Pseudomonas species using computerized methods, p.1–18.In R. G. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied and environmental microbiology . Blackwell Scientific Publications, Oxford, England.
- 11.Cowan, S. T., K. J. Steel, G. I. Barrow, and R. K. A. Feltham. 1993. Characters of Gram-negative bacteria, p.94–150. In G. I. Barrow and R. K. A. Feltham (ed.), Cowan and Steel’s manual for the identification of medical bacteria, 3rd ed. Cambridge University Press, London, United Kingdom.
- 12.Dalgaard, P. 2000. Fresh and lightly preserved seafood, p.110–139. In C. M. D. Man and A. A. Jones (ed.), Shelf-life evaluation of foods . Aspen Publishers, Gaithersburg, Md.
- 13.Davidson, C. M., and F. Cronin. 1973. Medium for the selective enumeration of lactic acid bacteria from foods. Appl. Environ. Microbiol. 26:439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vos, P., A. Van Landschoot, P. Segers, R. Tytgat, M. Gillis, M. Bauwens, R. Rossau, M. Goor, B. Pot, K. Kersters, P. Lizzaraga, and J. De Ley. 1989. Genotyping relationships and taxonomic localization of unclassified Pseudomonas and Pseudomonas-like strains by deoxyribonucleic acid: ribosomal ribonucleic acid hybridization. Int. J. Syst. Bacteriol. 39:35–49. [Google Scholar]
- 15.Drosinos, E. H. 1994. Microbial associations of minced lamb and their ecophysiological attributes. Ph.D. thesis. University of Bath, Bath, United Kingdom.
- 16.Drosinos, E. H., and R. G. Board. 1994. Metabolic activities of pseudomonads in batch cultures in extract of minced lamb. J. Appl. Bacteriol. 77:613–620. [DOI] [PubMed] [Google Scholar]
- 17.Eichholz, W. 1902. Erdbeerbacillus (Bacterium fragi). Zentbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 2 9:425–428. [Google Scholar]
- 18.Falkow, S. 1958. Activity of lysine decarboxylase as an aid in the identification of salmonellae and shigellae. Am. J. Clin. Pathol. 29:598. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Lopez, M. L., M. Prieto, and A. Otero. 1998. The physiological attributes of Gram-negative bacteria associated with spoilage of meat and meat products, p.1–28. In A. Davies and R. Board (ed.), The microbiology of meat and poultry . Blackie Academic and Professional, London, United Kingdom.
- 20.Gennari, M., and F. Dragotto. 1992. A study of the incidence of different fluorescent Pseudomonas species and biovars in the microflora of fresh and spoiled meat and fish, raw milk, cheese, soil and water. J. Appl. Bacteriol. 72:281–288. [DOI] [PubMed] [Google Scholar]
- 21.Gennari, M., S. Tomaselli, and V. Cotrona. 1999. The microflora of fresh and spoiled sardines (Sardinapilchardus) caught in Adriatic (Mediterranean) Sea and stored in ice. Food Microbiol. 16:15–28. [Google Scholar]
- 22.Gram, L., and H. H. Huss. 1996. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 33:121–137. [DOI] [PubMed] [Google Scholar]
- 23.Gram, L., C. Wedell-Neegaard, and H. H. Huss. 1990. The bacteriology of fresh and spoiling Lake Victorian Nile perch (Lates niloticus). Int. J. Food Microbiol. 10:303–316. [DOI] [PubMed] [Google Scholar]
- 24.Gregersen, T. 1978. Rapid method for distinction of Gram-negative and Gram-positive bacteria. Eur. J. Appl. Microbiol. 5:123–127. [Google Scholar]
- 25.Grothues, D., and B. Tummler. 1991. New approaches in genome analysis by pulse-field gel electrophoresis: application to the analysis of Pseudomonas species. Mol. Microbiol. 5:2763–2776. [DOI] [PubMed] [Google Scholar]
- 26.Hoffle, M. G. 1992. Rapid genotyping of pseudomonads by using low-molecular-weight RNA profiles, p.116–126. In E. Galli, S. Silver, and B. Witholt (ed.),Pseudomonas. American Society for Microbiology, Washington, D.C.
- 27.Hugh, R., and E. Leifson. 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative bacteria. J. Bacteriol. 66:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussong, R. V., H. F. Long, and B. W. Hammer. 1937. Classification of the organisms important in dairy products. Iowa Exp. Stn. Res. Bull. 225:119–136. [Google Scholar]
- 29.Johnsen, K., S. Andersen, and C. Jacobsen. 1996. Phenotypic and genotypic characterization of phenanthrene-degrading fluorescent Pseudomonas biovars. Appl. Environ. Microbiol. 62:3818–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersters, K., W. Ludwig, M. Vancanneyt, P. De Vos, M. Gillis, and K. H. Schleifer. 1996. Recent changes in the classification of pseudomonads: an overview. Syst. Appl. Microbiol. 19:465–477. [Google Scholar]
- 31.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301. [PubMed] [Google Scholar]
- 32.Koutsoumanis, K., and G.-J. E. Nychas. 1999. Chemical and sensory changes associated with microbial flora of Mediterranean Boque (Boops boops) stored aerobically at 0, 3, 7, and 10oC. Appl. Environ. Microbiol. 65:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs, N. 1956. Identification of Pseudomonas pyocyanea by oxidase reaction. Nature 178:703–707. [DOI] [PubMed] [Google Scholar]
- 34.Lahellec, C., and P. Colin. 1981.Pseudomonas as spoilage agents in poultry: observations concerning strains isolated at different stages and during storage, p.269–274. In T. A. Roberts, G. Hobbs, J. H. B. Christian, and N. Skovgaard (ed.), Psychrotrophic micro-organisms in spoilage and pathogenicity, vol. 25. Academic Press Ltd., London, United Kingdom.
- 35.Lebert, I., C. Begot, and A. Lebert. 1998. Growth of Pseudomonas fluorescens and Pseudomonas fragi in a meat medium as affected by pH (5.8–7.0), water activity (0.97–1.00) and temperature (7–25). Int. J. Food Microbiol. 39:53–60. [DOI] [PubMed] [Google Scholar]
- 36.Lehane, L., and J. Olley. 2000. Histamine fish poisoning revisited. Int. J. Food Microbiol. 58:1–37. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig, W., S. Dorn, N. Springer, et al. 1994. PCR-based preparation of 23S rRNA-targeted group-specific polynucleotide probes. Appl. Environ. Microbiol. 60:3236–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandel, M. 1966. Deoxyribonucleic acid base composition in the genus Pseudomonas. J. Gen. Microbiol. 43:272–292. [DOI] [PubMed] [Google Scholar]
- 39.Mayfield, C. I., and W. E. Innis. 1977. A rapid, simple method for staining bacterial flagella. Can. J. Microbiol. 23:1311–1313. [DOI] [PubMed] [Google Scholar]
- 40.Mead, G. C., and B. W. Adams. 1977. A selective medium for the rapid isolation of Pseudomonas associated with poultry meat spoilage. Br. Poult. Sci. 18:661–670. [DOI] [PubMed] [Google Scholar]
- 41.Molin, G., and A. Ternstrom. 1982. Numerical taxonomy of psychrotrophic pseudomonads. J. Gen. Microbiol. 128:1249–1264. [DOI] [PubMed] [Google Scholar]
- 42.Molin, G., and A. Ternstrom. 1986. Phenotypically based taxonomy of psychrotrophic Pseudomonas isolated from spoiled meat, water and soil. Int. J. Syst. Bacteriol. 36:257–274. [Google Scholar]
- 43.Molin, G., A. Ternstrom, and J. Ursing. 1986.Pseudomonas lundensis, a new bacterial species isolated from meat. Int. J. Syst. Bacteriol. 36:339–342. [Google Scholar]
- 44.Moore, W. E. C., E. P. Cato, and L. V. H. Moore. 1985. Index of bacterial and yeast nomeclatural changes published in the International Journal of Systematic Bacteriology since the 1980 Approved Lists of Bacterial Names (1 January 1980 to 1 January 1985). Int. J. Syst. Bacteriol. 35:382–407. [Google Scholar]
- 45.Mossel, D. A. A., and M. Ingram. 1955. The physiology of microbial spoilage of foods. J. Appl. Bacteriol. 18:232–268. [Google Scholar]
- 46.Nychas, G. J. E., V. M. Dillon, and R. G. Board. 1988. Glucose the key substrate in the microbiological changes occurring in meat and certain meat products. Biotechnol. Appl. Biochem. 10:203–231. [PubMed] [Google Scholar]
- 47.Palleroni, N. J. 1984. Genus I.Pseudomonas, p.141–199. In N. R. Krieg and J. G. Holt (ed.), Bergey’s manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 48.Palleroni, N. J. 1992. Introduction to the family Pseudomonadaceae, p.3071–3085. In A. H. Balows, G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 160. Springer-Verlag, New York, N.Y.
- 49.Palleroni, N. J. 1992. Human- and animal-pathogenic Pseudomonads, p.3086–3103. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 161. Springer-Verlag, New York, N.Y.
- 50.Palleroni, N. J., R. Kunisawa, R. Contopoulou, and M. Doudoroff. 1973. Nucleic acid homologies in the genus Pseudomonas. Int. J. Syst. Bacteriol. 23:333–339. [Google Scholar]
- 51.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints. Chemical methods, p.493–521. In M. Goodfellow and A. G. O’Donnell (ed.), Prokaryotic systematics . John Wiley and Sons, Chichester, United Kingdom.
- 52.Prieto, M., M. R. Garcia-Armesto, M. L. Garcia-Lopez, C. Alonso, and A. Otero. 1992. Species of Pseudomonas obtained at 7° C and 30° C during aerobic storage and lamb carcasses. J. Appl. Microbiol. 73:317–323. [DOI] [PubMed] [Google Scholar]
- 53.Rowe, M. T., and B. Finn. 1991. A study of Pseudomonas fluorescens biovars using the Automated Microbiology Identification System (AMBIS). Lett. Appl. Microbiol. 13:238–242. [Google Scholar]
- 54.Shaw, B. G., and J. B. Latty. 1982. A numerical taxonomic study of Pseudomonas strains from spoiled meat. J. Appl. Bacteriol. 52:219–228. [DOI] [PubMed] [Google Scholar]
- 55.Sierra, G. 1957. A simple method for the detection of lipolytic activity of microorganisms and some observations on the substrates. Antonie Leeuwenhoek 23:15–22. [DOI] [PubMed] [Google Scholar]
- 56.Sorensen, J., J. Skouv, A. Jorgensen, and O. Nybroe. 1992. Rapid identification of environmental isolates of Pseudomonas aeruginosa,P.fluorescens and P.putida by SDS-PAGE analysis of whole-cell protein patterns. FEMS Microbiol. Ecol. 101:41–50. [Google Scholar]
- 57.Stanbridge, L. H., and A. R. Davies. 1998. The microbiology of chill-stored meat, p.174–212. In A. Davies and R. Board (ed.), The microbiology of meat and poultry . Blackie Academic and Professional, London, United Kingdom.
- 58.Stanier, R. Y., N. J., Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159–271. [DOI] [PubMed] [Google Scholar]
- 59.Stead, D. E. 1992. Grouping of plant-pathogenic and some other Pseudomonas spp. by using cellular fatty acid profiles. Int. J. Syst. Bacteriol. 42:281–295. [Google Scholar]
- 60.Stenstrom, I.-M., and G. Molin. 1990. Classification of the spoilage flora of fish, with special reference to Shewanella putrefaciens. J. Appl. Bacteriol. 68:601–618. [DOI] [PubMed] [Google Scholar]
- 61.Thornley, M. J. 1960. The differentiation of Pseudomonas from other Gram-negative bacteria on the basis of arginine metabolism. J. Appl. Bacteriol. 23:37–52. [Google Scholar]
- 62.Ursing, J. 1986. Similarities of genome deoxyribonucleic acids of pseudomonas strains isolated from meat. Curr. Microbiol. 13:7–10. [Google Scholar]
- 63.Vancanneyt, M., U. Torck, D. Dewettinck, M. Vaerewijck, and K. Kersters. 1996. Grouping of Pseudomonads by SDS-PAGE of whole-cell proteins. Syst. Appl. Microbiol. 19:556–568. [Google Scholar]
- 64.Vauterin, L., J. Swing, and K. Kersters. 1993. Protein electrophoresis and classification, p.251–280. In M. Goodfellow and A. G. O’Donnell (ed.), Handbook of new bacterial systematics . Academic Press Ltd., London, United Kingdom.
- 65.Willems, A., P. De Vos, M. Gillis, and K. Kersters. 1992. Towards an improved classification of Pseudomonas, p.21–44. In R. G. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied and environmental microbiology . Blackwell Scientific Publications, Oxford, England.
- 66.Worm, J., L. E. Jensen, T. S. Hansen, M. Sondergaard, and O. Nybroe. 2000. Interactions between proteolytic and non-proteolytic Pseudomonas fluorescens affect protein degradation in a model community. Microb. Ecol. 32:103–109. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto, S., H. Kasai, D. L. Arnold, R. W. Jackson, A. Vivian, and S. Harayama. 2000. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology 146:2385–2394. [DOI] [PubMed] [Google Scholar]