Abstract
Sixteen type 1 poliovirus strains were isolated from a sewage disposal plant located downstream of the Oyabe River in Japan between October 1993 and September 1995. The isolates were intratypically differentiated as vaccine-derived strains. Neutralizing antigenicity analysis with monoclonal antibodies and estimation of neurovirulence by mutant analysis by PCR and restriction enzyme cleavage (MAPREC) were performed for 13 type 1 strains of these isolates. The isolates were classified into three groups. Group I (five strains) had a variant type of antigenicity and neurovirulent phenotype. Group II (four strains) had the vaccine type of antigenicity and neurovirulent phenotype. Group III (four strains) had the vaccine type of antigenicity and an attenuated phenotype. Furthermore, it was demonstrated that the virulent isolates were neutralized by human sera obtained after oral poliomyelitis vaccine (OPV) administration, and the sera of rats immunized with inactivated poliovirus vaccine. Although vaccination was effective against virulent polioviruses, virulent viruses will continue to exist in the environment as long as OPV is in use.
Oral poliomyelitis vaccine (OPV) is considered the major tool for worldwide eradication of poliomyelitis, and it has been used in many countries, including Japan. OPV is regarded as one of the safest vaccines in current use; however, some paralytic cases have been reported to have occurred in vaccinees or in individuals who have been in contact with vaccinees (21). Moreover, as long as OPV is in use, polioviruses will continue to exist in the environment. Recently, the number of countries using inactivated poliovirus vaccine (IPV) has increased to solve these problems (20).
The poliovirus has a genome of single-stranded RNA, which is known to be highly mutable during replication (11, 28). It was demonstrated that neurovirulence increased when the following changes took place in base positions of the viruses: in type 1 viruses, position 480 in the 5′ noncoding region changed from G to A (12, 15); position 525, which is base paired with position 480 in a stem and loop structure of the F-domain (26), changed from U to C (24); for type 2, position 481 changed from A to G (17, 22); and for type 3, position 472 changed from U to C (9, 29). Furthermore, Chumakov et al. (5, 6) designed the method of mutant analysis by PCR and restriction enzyme cleavage (MAPREC) to estimate the ratio of viruses containing genomes of a virulent nature in a vaccine virus population. MAPREC can be used for quality control of OPV production and surveillance of virus isolates.
In October 2000, the eradication of wild-type poliovirus in the Western Pacific Region that contains Japan was declared by the World Health Organization (WHO). In Japan, the wild-type strain was isolated in one patient with poliomyelitis in 1980; since then, no wild-type strain has been isolated from patients with poliomyelitis.
Studying the ecology of polioviruses excreted from the human gut into the environment is important for the final phase of polio eradication. Over the past few years, several studies have been made on polioviruses in the environment (23, 25, 27). In Japan, investigations of polioviruses in the environment have been carried out regularly since 1979 in Toyama Prefecture, and a total of 78 poliovirus strains (16 type 1 strains, 31 type 2 strains, and 31 type 3 strains) were isolated from environmental water samples between October 1993 and September 1995 (18). Furthermore, Yoshida et al. (32) reported that 55% of the type 3 poliovirus strains which were isolated from nature in Toyama Prefecture expressed a virulent phenotype.
In this study, we performed neutralizing antigenicity analysis with neutralizing monoclonal antibodies (MAbs) and estimation of neurovirulence by MAPREC in the 13 type 1 strains from a sewage in Toyama Prefecture. As a result, nine strains were estimated to have the neurovirulent phenotype, and a part of the neutralizing antigenic site of five of the nine strains has altered. Furthermore, neutralizing activities of human sera obtained after OPV administration and rat sera obtained from animals immunized with IPV against the neurovirulent isolates were analyzed to evaluate the effectiveness of vaccination against the environmental polioviruses.
MATERIALS AND METHODS
Virus isolation.
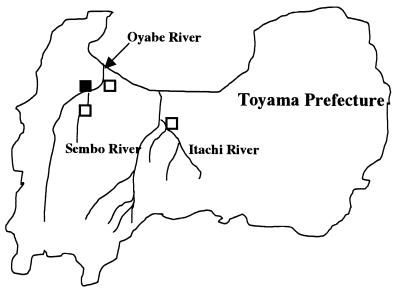
Sampling was performed twice monthly between October 1993 and September 1995 at a sewage disposal plant located downstream of the Oyabe River and at Oyabe, Itachi, and Sembo river monitoring points in Toyama Prefecture (18), as shown in Fig. 1. The collected samples were concentrated by the filter adsorption and elution method described previously (18). The concentrates were inoculated into tube cultures of rhabdomyosarcoma (RD), primary monkey kidney (MK), or Vero cells and incubated at 36°C in stationary culture conditions.
FIG. 1.
Geographic distribution of four sampling points in Toyama Prefecture in Japan. The symbols indicate three sampling points along the Oyabe, Itachi, and Sembo rivers (□) and a sewage disposal plant located alongside the Oyabe River (▪).
Virus multiplication was checked by cytopathic effect (CPE). Isolates were identified by neutralizing test with type-specific antipoliovirus antisera, which were prepared by immunizing rabbits with the Sabin type 1, 2, or 3 strain. The neutralizing test was performed according to standard procedures (30). No type 1 poliovirus was isolated from the river water. Sixteen type 1 polioviruses were isolated from the sewage, and 13 of them were used in this study because of insufficient amounts of the others for analysis.
Determination of neutralizing antibody titers.
Neutralizing titers of Sabin type 1 strain-specific neutralizing MAb 8a027, which neutralizes Sabin type 1 strain but dose not neutralize Brunhilde, Brunenders, and Mahoney strains (13); anti-Sabin type 1 strain neutralizing MAb 8a057, which neutralizes Sabin type 1, Brunhilde, Brunenders strains, dose not neutralize Mahoney strain (13); and 8a141, which neutralizes Sabin type 1, Brunhilde, Brunenders, and Mahoney strains (13), against the 13 type 1 isolates were analyzed.
Twelve human sera obtained after administration of OPV to healthy 10- to 12-year-old children who had previously been vaccinated for polio and 12 rat sera obtained from animals immunized with IPV, which was preliminarily prepared from Sabin strains using Vero cell cultivation, administered intramuscularly twice at 3-week intervals, were used for determination of the neutralizing activity against three isolates, G4-2, G4-12, and G17-21. The neutralizing test was performed by the standard method recommended by WHO (31). Briefly, 50 μl of serial twofold MAbs, human sera, or immunized rat sera dilution series were prepared, in duplicate, in Eagle’s minimal essential medium (MEM) supplemented with 2% fetal calf serum (FCS) in a 96-well microtiter plate. Then 50 μl of 100 50% cell culture infectious doses (CCID50) of the each isolate, Sabin type 1 vaccine strain F113, or type 1 wild strain Mahoney was added to each well. After incubation at 36°C for 2 h, 100 μl of a cell suspension containing 104 HEp2-C cells in MEM supplemented with 5% FCS was added to each well. The plates were then scored for CPE after 7 days of incubation at 36°C in a CO2 atmosphere. The calculation of the neutralizing titer of each sample was determined by the Karber method (30).
MAPREC.
To determine specific mutations at genome positions 480 and 525, MAPREC was performed according to the methods of Chumakov et al. (5, 7) and Rezapkin et al. (24). Briefly, viral RNA was extracted directly from 0.5 ml of virus suspension by phenol extraction with 1% sodium dodecyl sulfate (SDS). cDNA was synthesized with Moloney murine leukemia virus reverse transcriptase (Gibco-BRL) and random hexadeoxynucleotide primers (Takara, Tokyo, Japan).
PCR amplification using sense and antisense primers as described previously (7, 24) and Taq DNA polymerase (AmpliTaq; Perkin-Elmer/Cetus) was conducted so as to create recognition sites for restriction enzyme AluI (for detection of virulence-associated base A at position 480) and ScrF1 (for detection of virulence-associated base C at position 525). After treatment of the amplified DNA product with the restriction enzyme, the digested material was separated by electrophoresis in a polyacrylamide gel. The proportion of mutational change in a viral genome estimated by MAPREC (percent 480-A and 525-C) was calculated by measuring radioactivity in counts per minute (cpm) of digested and undigested DNA bands with AluI or ScrF1, using the equation {digested DNA (cpm)/[digested DNA (cpm) + undigested DNA (cpm)]}. Sabin type 1 vaccine virus F113 and type 1 wild strain Mahoney were used as reference strains for the attenuated and virulent phenotypes, respectively.
Neurovirulence test.
Transgenic PVR-Tg21 mice, which carry the human poliovirus receptor and are susceptible to poliovirus (16), at the age of 8 to 10 weeks were used for the neurovirulence test. F113 and five isolates, G4-2, G4-12, G17-21, G16-1, and G35-10, were serially diluted 10-fold, and 5 μl of each dilution was inoculated into the spinal cord of 10 mice per dilution. Inoculated mice were observed for 14 days. The 50% paralysis dose (PD50) and 50% lethal dose (LD50) were calculated by the Probit method (31).
RESULTS
Neutralizing antigenicity analysis of isolates.
Collection dates and culture history of the 13 isolates are shown in Table 1. Nucleotide sequences analysis of the 13 isolates in the VP1 and VP3 regions was performed previously by Matsuura et al. (18). The nucleotide sequences of three strains, G16-1, G17-21, and G35-10, were identical to those of Sabin type 1, and then, although the other 10 strains had one to six point mutations in these regions, these 10 strains were identified as vaccine variants that had less than 1.4% nucleotide divergence from the Sabin type 1 strain (18).
TABLE 1.
Characterization of type 1 poliovirus isolates from a sewage disposal plant
| Strain | Collection date | Culture historya | Neutralizing antibody titer with MAb:
|
% 480-A + 525-C by MAPREC | ||
|---|---|---|---|---|---|---|
| 8a027 | 8a057 | 8a141 | ||||
| References | ||||||
| F113 | >262,144 | 65,536 | >262,144 | 0.810 | ||
| Mahoney | <64 | <64 | >262,144 | 81.1 | ||
| Isolates | ||||||
| G3-11 | Nov. 1993 | Vero/4 | >262,144 | 16,384 | >262,144 | 91.8 |
| G4-2 | Nov. 1993 | Vero/3 | 256 | 262,144 | >262,144 | 89.3 |
| G4-12 | Nov. 1993 | Vero/3 | 256 | 1,024 | 65,536 | 92.2 |
| G4-16 | Nov. 1993 | RD/2, Vero/2 | 65,536 | 65,536 | 262,144 | 0.791 |
| G16-1 | May 1994 | Vero/3 | >262,144 | 65,536 | >262,144 | 0.371 |
| G16-6 | May 1994 | MK/1, Vero/1 | >262,144 | 65,536 | 262,144 | 0.974 |
| G17-21 | June 1994 | MK/1, Vero/1 | >262,144 | 262,144 | >262,144 | 93.7 |
| G18-5 | June 1994 | Vero/3 | >262,144 | <64 | 65,536 | 93.8 |
| G26-11 | Oct. 1994 | Vero/2 | >262,144 | 262,144 | 262,144 | 93.7 |
| G28-3 | Nov. 1994 | Vero/2 | 256 | 16,384 | >262,144 | 83.1 |
| G28-9 | Nov. 1994 | Vero/2 | <64 | 4,096 | >262,144 | 92.8 |
| G35-10 | Mar. 1995 | Vero/2 | >262,144 | 262,144 | >262,144 | 0.485 |
| G42-7 | June 1995 | Vero/2 | >262,144 | 65,536 | >262,144 | 90.2 |
Cell type/number of passages.
The neutralizing antigenicity of the isolates was analyzed with the Sabin type 1 strain-specific neutralizing MAb 8a027 and the anti-Sabin type 1 strain neutralizing MAbs 8a057 and 8a141. As shown in Table 1, neutralizing antibody titers of MAb 8a027 against strains G4-2, G4-12, G28-3, and G28-9 showed a marked decrease compared with Sabin type 1 vaccine virus F113, and MAb 8a057 showed a reduction of neutralizing antibody titers against isolates G4-12, G18-5, and G28-9. It is considered that the neutralizing antigenic site of G4-2, G4-12, G18-5, G28-3, and G28-9 which is recognized by MAb 8a027 and/or 8a057 was altered from the Sabin type 1 vaccine virus. On the other hand, MAb 8a141 easily neutralized all isolates (Table 1).
Estimation of neurovirulence by MAPREC.
The neurovirulence of the isolates was analyzed by the MAPREC test, which was used to estimate the ratio of viruses containing genes of a virulent nature in a virus population. In the case of type 1 poliovirus, the stipulated cut-off of the percentage of 480-A + 525-C for passing or failing the monkey neurovirulence test is approximately 5% (24). As shown in Table 1, the 480-A + 525-C content of nine strains, G3-11, G4-2, G4-12, G17-21, G18-5, G26-11, G28-3, G28-9, and G42-7, was demonstrated to be over 80%, which was almost the same level as for type 1 wild strain Mahoney. These strains are expected to have extremely high neurovirulence. On the other hand, the percentages of 480-A + 525-C in G4-16, G16-1, G16-6, and G35-10 were under 1%, the same as for type 1 vaccine virus F113. No intermediate type was found in the isolates.
Classification of isolates.
On the basis of antigenicity analysis using neutralizing MAbs and neurovirulence analysis by MAPREC, the isolates were classified into three groups as shown in Table 2. Strains G4-2, G4-12, G18-5, G28-3, and G28-9 belong to group I, which has a variant type of neutralizing antigenicity and neurovirulent phenotype. Strains G3-11, G17-21, G26-11, and G42-7 belong to group II, and strains G4-16, G16-1, G16-6, and G35-10 to group III; they all have the vaccine type of neutralizing antigenicity, but whereas the strains of group II show a neurovirulent phenotype, those of group III show an attenuated phenotype.
TABLE 2.
Grouping of isolates by neutralizing antigenicity and neurovirulence
| Category | Neutralizing antigenicity | Neuro-virulence | Strains |
|---|---|---|---|
| Group I | Variant | Virulent | G4-2, G4-12, G18-5, G28-3, G28-9 |
| Group II | Vaccine | Virulent | G3-11, G17-21, G26-11, G42-7 |
| Group III | Vaccine | Attenuated | G4-16, G16-1, G16-6, G35-10 |
Neurovirulence test in PVR-Tg21 mice.
In order to measure the actual neurovirulence of the isolates, isolates derived from each group as shown in Table 2 were inoculated into the spinal cord of PVR-Tg21 transgenic mice. The neurovirulence of five strains: G4-2 and G4-12 belonging to group I, G17-21 from group II, and G16-1 and G35-10 from group III was evaluated by PD50 and LD50 as shown in Table 3. Neurovirulences of strains G4-2, G4-12, and G17-21, which had >80% 480-A + 525-C, were significantly higher than for Sabin type 1 vaccine virus F113. Neurovirulences of strains G16-1 and G35-10, which had approximately the same percentage of 480-A + 525-C as F113, were shown to be attenuated. Thus, the neurovirulence of these isolates in PVR-Tg21 mice was demonstrated to correlate closely with the results of MAPREC.
TABLE 3.
Neurovirulence of type 1 isolates in PVR-Tg21 transgenic mice
| Virus | % 480-A + 525-C by MAPREC | Neurovirulence in PVR-Tg21 micea at inoculum (log10 CCID50):
|
PD50 (log10 CCID50) | LD50 (log10 CCID50) | ||
|---|---|---|---|---|---|---|
| 1.0 | 2.0 | 3.0 | ||||
| G4-2 | 89.3 | 1 (1)/10 | 6 (6)/10 | 10 (10)/10 | 1.69 | 1.69 |
| G4-12 | 92.2 | 0 (0)/10 | 6 (6)/10 | 10 (10)/10 | 1.79 | 1.79 |
| G17-21 | 93.7 | 2 (1)/10 | 5 (5)/10 | 8 (8)/10 | 1.83 | 1.97 |
| G16-1 | 0.371 | 0 (0)/10 | 4 (1)/10 | 6 (3)/10 | 2.48 | >3.84 |
| G35-10 | 0.485 | 0 (0)/10 | 2 (1)/10 | 9 (4)/10 | 2.35 | 3.30 |
| F113 | 0.810 | 0 (0)/10 | 3 (3)/10 | 9 (6)/10 | 2.25 | 2.62 |
Data are number of paralyzed mice (number of dead mice)/number of mice inoculated.
Neutralization of isolates by human and rat sera.
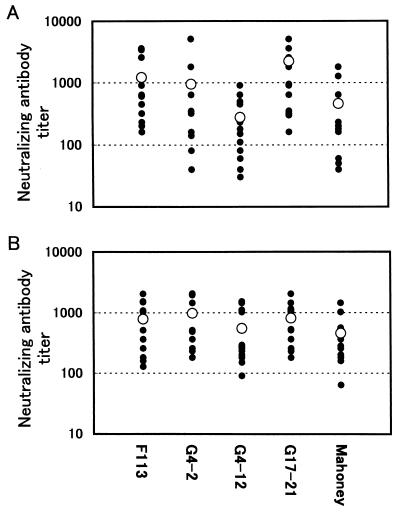
The neutralizing activity of human sera obtained after OPV administration against isolates belonging to group I or II was analyzed. As shown in Fig. 2A, all human sera used for the test showed neutralizing activity for the three strains G4-2, G4-12, and G17-21. However, the neutralizing activity for G4-12 was slightly lower than that for the other strains.
FIG. 2.
Neutralizing antibody titers of human and rat sera against virulent type 1 isolates. (A) Twelve human sera obtained after administration of OPV to healthy 10- to 12-year-old children who were previously vaccinated for polio and (B) 12 rat sera obtained from animals immunized with IPV intramuscularly twice were tested to determine the neutralizing activity against three isolates, G4-2, G4-12, and G17-21, the Sabin type 1 reference virus F113, and the virulent strain Mahoney. The symbols indicate neutralizing antibody titers (•) and mean titers (○).
The neutralizing activity of rat sera obtained after IPV immunization against the isolates was analyzed as well (Fig. 2B). It was demonstrated that the all rat sera easily neutralized strains G4-2, G4-12, and G17-21.
DISCUSSION
The neutralizing antigenicity and neurovirulence of 13 type 1 isolates obtained from a sewage disposal plant located along the Oyabe River in Toyama Prefecture between October 1993 and September 1995 were analyzed. OPV administration is carried out in this region in May and October each year. These isolates were considered to be viruses excreted from humans inoculated with OPV, based on nucleotide sequencing analysis and isolation time (18). However, the survival period of the viruses in the environment is unclear. It is possible that the isolates were continuously excreted from circulation in the human community.
The neurovirulence of the isolates was analyzed by MAPREC and neurovirulence test in PVR-Tg21 transgenic mice. Poliovirus-susceptible PVR-Tg21 mice, which were developed by Koike et al. (16), are expected to be useful for neurovirulence tests on the safety of OPV production. MAPREC was established by Chumakov et al. (5–7) to estimate the ratio of viruses containing genes of a virulent nature in a vaccine virus population. In the case of type 1 viruses, it has been reported that the percent 480-A + 525-C correlated with neurovirulence (13, 24), and the culture temperature, the cell substrate, and the multiplicity of infection influence the percentage when viruses are cultured (7, 13, 14, 24).
In the present study, the possibility that mutations in the viral genes influenced neurovirulence caused during the cultivation of virus for isolation is unlikely, because the passage conditions of isolates did not always correlate with the content of 480-A + 525-C. For instance, strains G4-2, G4-12, G16-1, and G18-5 were passaged three times in Vero cells at 36°C, but G16-1 had an attenuated phenotype with less than 1% 480-A + 525-C, while the other strains showed a virulent phenotype (Table 1). Similarly, strains G16-6 and G17-21 were passaged two times in MK and Vero cells, but one showed an attenuated and the other a virulent phenotype (Table 1). Furthermore, there has been no report that the percentage of MAPREC increased rapidly to over 80% during only two or three passages at 36°C.
In this study, the environmental strains were classified into three groups, as shown in Table 2, by neutralizing antigenic analysis and MAPREC. The excretion of poliovirus from healthy children following the administration of OPV continues for approximately 2 months (1, 2). It has been reported that the excreted viruses have a back mutation which develops during replication in the human (2, 10, 19). Dunn et al. (8) revealed that mutation rate at nucleotide position 480 of type 1 excreted viruses was approximately 50 to 80% in 4 weeks after first-time OPV administration. Furthermore, it has been reported that the period of excretion from individuals who were immunized with OPV previously or infected naturally was shorter than that of susceptible individuals (1, 2).
On the basis of these reports, it is considered that the strains belonging to group I were continuously excreted for a long time from susceptible individuals and/or circulated in the community, the strains belonging to group II were excreted from susceptible humans over a shorter time, while the strains belonging to group III might have been excreted promptly from previously immunized individuals. Research into the mutations in viruses excreted from humans after administration of OPV is required to certify this hypothesis. Although there is a little chance that individuals come into direct contact with sewage, it is quite likely that the environmental polioviruses infect humans.
The most important point is whether we can be protected from the viruses with a virulent phenotype. Our results demonstrated that human sera after administration of OPV and the sera of rats immunized with IPV easily neutralized the isolates which had a virulent phenotype. As long as vaccination is continued, it is expected that we will be safe from the wild-type and environmental polioviruses. When vaccination coverage decreases after wild-type poliovirus eradication, outbreaks of poliomyelitis caused by the environmental polioviruses are a concern. Actually, a current outbreak of poliomyelitis by vaccine-derived type 1 poliovirus has occurred in the Dominican Republic and Haiti (3), which have been free of wild-type poliovirus since 1991. Furthermore, it has been reported that 32 polio cases associated with vaccine-derived poliovirus type 2 were found in Egypt (4). These incidents will influence vaccine strategy for polio eradication in future. As long as OPV is in use, it will be difficult to eradicate polioviruses from the environment. The introduction of IPV will be necessary for the final phase of polio eradication.
Acknowledgments
We thank B. Simizu (Japan Poliomyelitis Research Institute), T Kitamura (Toyama Institute of Health), and T Miyamura (National Institute of Infectious Diseases) for helpful discussions and K. Irisawa for assistance in intraspinal inoculation of PVR-Tg21 mice.
REFERENCES
- 1.Alexander, J. P., Jr., H. E. Gary, Jr., and M. A. Pallansch. 1997. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J. Infect. Dis. 175(Suppl. 1):S176–182. [DOI] [PubMed] [Google Scholar]
- 2.Benyesh-Melnick, M., J. L. Melnic, W. E. Rawls, I. Wimberly, J. B. Oro, E. Ben-Porath, and V. Rennick. 1967. Studies of the immunogenicity, communicability and genetic stability of oral polio vaccine administration during the winter. Am. J. Epidemiol. 86:112–136. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Outbreak of poliomyelitis—Dominican Republic and Haiti, 2000. Morb. Mortal. Wkly. Rep. 49:1094, 1103. [PubMed]
- 4.Centers for Disease Control and Prevention. 2001. Circulation of a type 2 vaccine-derived poliovirus—Egypt, 1982–1993. Morb. Mortal. Wkly. Rep. 50:41–42, 51. [PubMed]
- 5.Chumakov, K. M., L. B. Powers, K. E. Noonan, I. B. Roninson, and I. S. Levenbook. 1991. Correlation between amount of virus with altered nucleotide sequence and the monkey test for acceptability of oral poliovirus vaccine. Proc. Natl. Acad. Sci. USA 88:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chumakov, K. M., L. P. Norwood, M. L. Parker, E. M. Dragunsky, Y. Ran, and I. S. Levenbook. 1992. RNA sequence variants in live poliovirus vaccine and their relation to neurovirulence. J. Virol. 66:966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chumakov, K. M., E. M. Dragunsky, L. P. Norwood, M. P. Douthitt, Y. Ran, R. E. Taffs, J. Ridge, and I. S. Levenbook. 1994. Consistent selection of mutations in the 5′-untranslated region of oral poliovirus vaccine upon passaging in vitro. J. Med. Virol. 42:79–85. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, G., N. T. Begg, N. Cammack, and P. D. Minor. 1990. Virus excretion and mutation by infants following primary vaccination with live oral polio vaccine from two sources. J. Med. Virol. 32:92–95. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. M. A., G. Dunn, P. D. Minor, G. C. Schild, A. J. Cann, G. Stanway, J. W. Almond, K. Currey, and J. V. Maizel. 1985. Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 polio vaccine genome. Nature 314:548–550. [DOI] [PubMed] [Google Scholar]
- 10.Georgescu, M. M., J. Balanant, A. Macadam, D. Otelea, M. Combiescu, A. A. Combiscu, R. Crainic, and F. Delpeyroux. 1997. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 71:7758–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland, J., K. Spindler, F. Horodyski, E. Grabau, S. Nichol, and S. Vande Pol. 1982. Rapid evolution of RNA genomes. Science 215:1577–1585. [DOI] [PubMed] [Google Scholar]
- 12.Horie, H., S. Koike, T. Kurata, Y. Sato-Yoshida, I. Ise, Y. Ota, S. Abe, K. Hioki, H. Kato, C. Taya, T. Nomura, S. Hashizume, H. Yonekawa, and A. Nomoto. 1994. Transgenic mice carrying the human poliovirus receptor: new animal model for study of poliovirus neurovirulence. J. Virol. 68:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horie, H., M. Sato-Miyazawa, Y. Ota, K. Wakabayashi, T. Doi, K. Yoshizawa, Y. Doi, and S. Hashizume. 1999. Detection of mutants in polio vaccine viruses using pooled antipoliovirus monoclonal antibodies. Biologicals 27:217–226. [DOI] [PubMed] [Google Scholar]
- 14.Horie, H., M. Miyazawa, Y. Ota, K. Wakabayashi, H. Yoshida, Y. Doi, S. Hashizume. 2001. Analysis of the accumulation of mutants in Sabin attenuated polio vaccine viruses passaged in Vero cells. Vaccine 19:1456–1459. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura, N., M. Kohara, S. Abe, T. Komatsu, K. Tago, M. Arita, and A. Nomoto. 1989. Determinants in the 5′ noncoding region of poliovirus Sabin 1 RNA that influence the attenuation phenotype. J. Virol. 63:1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike, S., T. Taya, T. Kurata, S. Abe, I. Ise, H. Yonekawa, and A. Nomoto. 1991. Transgenic mice susceptible to poliovirus. Proc. Natl. Acad. Sci. USA 88:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macadam, A. J., S. R. Pollard, G. Ferguson, G. Dunn, R. Skuce, J. W. Almond, and P. D. Minor. 1991. The 5′ noncoding region of the type 2 poliovirus vaccine strain contains determinants of attenuation and temperature sensitivity. Virology 181:451–458. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura, K., M. Ishikura, H. Yoshida, T. Nakayama, S. Hasegawa, S. Ando, H. Horie, T. Miyamura, and T. Kitamura. 2000. Assessment of poliovirus eradication in Japan: genomic analysis of the polioviruses isolated from river water and sewage in Toyama Prefecture. Appl. Environ. Microbiol. 66:5087–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulders, M. N., J. H. Reimerink, M. Stenvik, I. Alaeddionoglu, H. G. van der Avoort, T. Hovi, and M. P. Koopmans. 1999. A Sabin vaccine-derived field isolate of poliovirus type 1 displaying aberrant phenotypic and genetic features, including a deletion in antigenic site 1. J. Gen. Virol. 80:907–916. [DOI] [PubMed] [Google Scholar]
- 20.Murdin, A. D., L. Barreto, and S. Plotkin. 1996. Inactivated poliovirus vaccine: past and present experience. Vaccine 14:735–746. [DOI] [PubMed] [Google Scholar]
- 21.Nkowane, B. M., S. G. F. Wassilak, W. A. Orenstein, K. J. Bart, L. B. Schonberger, A. R. Hinman, and O. M. Kew. 1987. Vaccine-associated paralytic poliomyelitis, United States: 1973 through 1984. JAMA 257:1335–1340. [PubMed] [Google Scholar]
- 22.Pollard, S. R., G. Dunn, N. Cammack, P. D. Minor, and J. W. Almond. 1989. Nucleotide sequence of a neurovirulent variant of the type 2 oral poliovirus vaccine. J. Virol. 63:4949–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poyry, T., M. Stenvik, and T. Hovi. 1988. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl. Environ. Microbiol. 54:371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rezapkin, G. V., K. M. Chumakov, Z. Lu, Y., Ran, E. M. Dragunsky, and I. S. Levenbook. 1994. Microevolution of Sabin 1 strain in vitro and genetic stability of oral poliovirus vaccine. Virology 202:370–378. [DOI] [PubMed] [Google Scholar]
- 25.Shulman, L. M., Y. Manor, R. Handsher, F. Delpeyroux, M. J. Modonough, T. Halmut, I. Silberstein, J. Alfandari, J. Quay, T. Fisher, J. Robinov, O. M. Kew, R. Crainic, and E. Mendelson. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral polio vaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 38:3729–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner, M. A., V. R. Racaniello, G. Dunn, J. Cooper, P. D. Minor, and J. W. Almond. 1989. New model for the secondary structure of the 5′ noncoding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important for neurovirulence. J. Mol. Biol. 207:379–392. [DOI] [PubMed] [Google Scholar]
- 27.Tambini, G., J. K. Andrus, E. Marques, J. Boshell, M. Pallansch, C. A. de Quadros, and O. M. Kew. 1993. Direct detection of wild poliovirus circulation by stool surveys of healthy children and analysis of community wastewater. J. Infect. Dis. 168:1510–1514. [DOI] [PubMed] [Google Scholar]
- 28.Ward, C. D., M. A. M. Stockes, and J. B. Flanegan. 1988. Direct measurement of the poliovirus RNA polymerase error frequency in vitro. J. Virol. 62:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westrop, G. D., K. A. Wareham, D. M. Davis, G. Dunn, P. D. Minor, D. I. Magrath, F. Taffs, S. Marsden, M. A. Skinner, G. C. Schild, and J. W. Almond. 1989. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J. Virol. 63:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 1990. Manual for the virological investigation of poliomyelitis. World Health Organization, Expanded Programme on Immunization and Division of Communicable Diseases. W.H.O. publication no. W.H.O./EPI/CDS/POLIO/90.1. World Health Organization, Geneva, Switzerland.
- 31.World Health Organization. 1995. Manual of laboratory methods for potency testing of vaccines used in the W.H.O. Expanded Programme on Immunization. W.H.O. publication no. W.H.O./BLG/95.1. World Health Organization, Geneva, Switzerland.
- 32.Yoshida, H., H. Horie, K. Matsuura, and T. Miyamura. 2000. Characterization of polioviruses isolated from sewage and river water in Japan. Lancet 356:1461–1463. [DOI] [PubMed] [Google Scholar]