Abstract
Microbial communities in biofilms grown for 4 and 11 weeks under the flow of drinking water supplemented with 0, 1, 2, and 5 μg of phosphorus liter−1 and in drinking and warm waters were compared by using phospholipid fatty acids (PLFAs) and lipopolysaccharide 3-hydroxy fatty acids (LPS 3-OH-FAs). Phosphate increased the proportion of PLFAs 16:1ω7c and 18:1ω7c and affected LPS 3-OH-FAs after 11 weeks of growth, indicating an increase in gram-negative bacteria and changes in their community structure. Differences in community structures between biofilms and drinking and warm waters can be assumed from PLFAs and LPS 3-OH-FAs, concomitantly with adaptive changes in fatty acid chain length, cyclization, and unsaturation.
The availability of assimilable organic carbon often limits the growth of heterotrophic bacteria in drinking water (18, 42). However, within the northern boreal region in Finland, the assimilable organic carbon content has correlated poorly with microbial growth in drinking water. Instead, the regulating factor appears to be the amount of phosphorus present in water (21, 23). Similar results have been found in Norway and Japan (4, 34, 35). Biofilms have special importance for drinking water quality because they can provide protection for microbes against disinfection (16, 17), increase microbial growth (including coliforms) (6, 15), and induce scaling and corrosion in the pipes (5). In addition, biofilm growth and detachment are thought to account for a major number of the microbial cells present in drinking water (44).
Isolation of microbes can reveal only a small part of the diversity in microbial communities (46). In Finland, less than 0.5% of the microbes in groundwater were found to be culturable (22). The combined phospholipid fatty acid (PLFA) and lipopolysaccharide 3-hydroxy fatty acid (LPS 3-OH-FA) analyses have earlier been used to describe microbial community structures and biomass in soils (51, 52) and sediments (7). PLFAs have been studied from the biological filters used for drinking water treatment (25, 26, 27) and from water distribution system biofilms exposed to chlorine (36). Our aim was to use the PLFA and LPS 3-OH-FA analyses to determine whether the addition of phosphate would change the microbial community structure in drinking water biofilms. The microbial community structure in the biofilms was compared to that in drinking and warm waters.
The drinking water samples were from Kuopio waterworks in Finland, which processes bank-filtered lake water. The lake water was filtered through the banks to wells and further pumped to the waterworks for purification. Iron, manganese, and humic substances were coagulated with aluminum sulfate, followed by rapid sand filtration, and finally disinfected with sodium hypochlorite. The drinking water for biofilm experiments was not disinfected. The drinking water retention time was 1 to 2 days in pipelines before it arrived at the sampling point in the building. The warm water was heated from drinking water in the warm water circulation system of the building. Two drinking water (7°C) samples of 30.0 and 29.8 liters and warm water (45°C) samples of 29.2 and 28.2 liters were collected in May 1999 and filtered through 0.2-μm (pore size; diameter, 14.2 cm) nylon filters (Pall Europe, Ltd., Portsmouth, England) with filtration equipment (Sartorius SM 16274; Sartorius GmbH, Goettingen, Germany). The filter and retentate were frozen at −20°C and lyophilized (Edwards 4 K Modulyo Freeze Dryer; Edwards, Crawley, England). The amount of culturable heterotrophic bacteria in drinking water (March 2000) was determined on R2A agar (33). The total phosphorus content in drinking water was 2 μg liter−1, as determined by the ascorbic acid method (8, 23). The concentration of microbially available phosphorus was 0.14 μg liter−1, as determined by a bioassay in which the maximum growth of Pseudomonas fluorescens in water sample is converted to phosphorus content in water (19).
The glassware and the silicon and Teflon tubes used in biofilm experiments were washed with phosphate-free detergent (Deconex; Borer Chemie AG, Zuchwil, Switzerland), immersed in 2% HCl solution for 2 h, and then rinsed with deionized water (Millipore, Molsheim, France). All pieces of glassware were heated for 6 h at 550°C (Hobbyceram, Milan, Italy), and the silicon and Teflon tubes were sterilized at 120°C for 15 min. Biofilms were grown in the dark at 21 ± 2°C on glass slides (41.6 cm2) in glass chambers of 186.5 cm3. The drinking water was pumped (IPC-N-16 V1.32; Ismatec, Grattbugg-Zürich, Switzerland) through the chambers with the flow velocity of 0.5 ml min−1, with a water retention time of 6 h 13 min. A separate Na2HPO4 solution flow to the chamber was used for supplementation of 1, 2, and 5 μg of phosphorus liter−1. The glass slides were taken from the chambers after 4 or 11 weeks, rinsed gently with sterile water, and crushed with a stainless steel chisel. Biofilms were detached from glass slides in 30 ml of sterile ion-exchange-purified water by 5 min of sonication (40 kHz) (Finnsonic mO3, Lahti, Finland). The extract of 20 ml was filtered, and the filter and retentate were frozen at −20°C and lyophilized as described for the water samples.
For lipid analyses the glassware was heated at 550°C for 6 h. Duplicate filters without samples were used as references and analyzed identically to the filters with samples. Extraction and storage of lipids (−20°C) were done under a nitrogen atmosphere. Duplicate water and biofilm retentates on filters were extracted in 28.2 ml of chloroform–methanol–50 mM phosphate buffer (pH 7.4, 1:2:0.8 [vol/vol/vol]) by shaking for at least 2 h at 21 ± 2°C (2, 9, 47). For the quantification of phospholipids, diheptadecanoylphosphatidylcholine was added as an internal standard, and the samples were shaken for 5 min. The solvents were separated by centrifuging (2,000 × g), and the volumes were measured. Chloroform and buffer were added to obtain final ratios of chloroform-methanol-phosphate buffer (pH 7.4; 1:1:0.9 [vol/vol/vol]), and the samples were centrifuged for 10 min (2,000 × g). The solvent layer was evaporated to dryness in a centrifugal evaporator (Jouan RC10.10; Jouan S.A., Saint-Herblain, France). A glass column (height, 100 mm; inner diameter, 6 mm) was packed with 0.75 g of silicic acid (100 to 200 mesh size; Unisil; Clarkson Chemical, Williamsport, Pa.) activated at 120°C for 2 h and washed with chloroform. The lipid extract was applied in chloroform (three times, 100 μl each time) on the top of the column, and neutral lipids were eluted with 10 ml of chloroform, glycolipids were eluted with 20 ml of acetone, and phospholipids were eluted with 10 ml of methanol (9, 13). The phospholipid fraction was evaporated into dryness, and tridecanoic and nonadecanoic acid methyl esters were added as internal standards. Fatty acids were saponified, methylated, and extracted as methyl esters (38). The internal standard, 3-OH tridecanoic acid methyl ester, was added to the extraction residues, and LPS 3-OH-FAs were analyzed by the mild acid hydrolysis (11, 39). Fatty acids were analyzed with a Hewlett-Packard (Palo Alto, Calif.) model G1800A gas-liquid chromatograph equipped with a mass selective detector and an HP7673 automatic sampler. The gas-liquid chromatography conditions were as follows: HP-5 capillary column (30 m by 0.2 mm by 0.11 μm) coated with cross-linked 5% PhMe silicone; carrier gas, helium (1.0 ml min−1); injection, splitless; injector temperature, 250°C; and detector temperature, 270°C. The oven temperature was programmed to hold 50°C for 1 min and then increase 30°C min−1 up to 160°C and thereafter by 5°C min−1 up to 270°C. The PLFA samples were analyzed with the selected ion monitoring by monitoring the ions m/z 74 and 199. The latter ion, however, was m/z 268 for 16:1 acids, m/z 250 for cy-17:0, m/z 298 for i-18:0 and 18:0, m/z 294 for 18:2ω6c, m/z 264 for 18:1, m/z 312 for 10-Me-18:0 and 19:0, and m/z 278 for cy-19:0, and m/z 326 for 20:0 acids. In selected ion monitoring of LPS 3-OH-FA methyl esters, the ion monitored was m/z 103 (10). The fatty acid methyl ester peaks were identified by comparing their mass spectra and retention times with those of standards.
To calculate the calibration curves for the quantification of fatty acids, calibration standards were made with known ratios of bacterial fatty acids relative to the internal standard methyl nonadecanoate (19:0) (40). The standards contained fatty acids at four to five concentrations ranging from 0.02 to 2 nmol μl−1 for 16:0, with an internal standard amount of 68 pmol μl−1. The fatty acid content was defined as the sum of the fatty acid methyl esters. The PLFA content was converted to cell densities by using the following factors: on average, bacteria contain 100 μmol of PLFAs g−1 (dry weight), and 1 g of bacteria (dry weight) is equivalent to 2.0 × 1012 cells (dry weight) (1). The C18/C16 value was calculated as the percentage ratio of 18:0, 18:1ω7c, and 18:1ω9c to 16:0, 16:1ω5c, and 16:1ω7c. All results are presented as the mean ± the standard error. Principal component analyses (PCA) and Pearson correlation analyses were performed by using the SAS procedures PRINCOMP and CORR, respectively (User’s Guide, version 6, 4th ed.; SAS Institute, Cary, N.C.).
All reagents were from Merck (Darmstadt, Germany) except for solvents (Rathburn, Ltd., Peeblesshire, United Kingdom), 3-hydroxytridecanoic acid methyl ester (Larodan AB, Malmö, Sweden), other fatty acid standards (Sigma, St. Louis, Mo.), NaOH (FF-Chemicals, Yli-Ii, Finland), HCl (Riedel-de Haën, Seelze, Germany), and acetylchloride (Fluka, Buchs, Switzerland).
The PLFA analyses identified 21 to 22 fatty acids, which were most commonly saturated and unsaturated straight-chain acids, such as 16:0, 18:0, 18:1ω7c, and 18:1ω9c in biofilms and 16:0, 18:0, 16:1ω7c and 18:1ω7c in waters (Table 1). The LPS 3-OH-FAs detected were 3-OH-10:0, 3-OH-12:0, and 3-OH-14:0. These fatty acids are characteristic of gram-negative bacteria (48), which have been shown by PLFA and culture results to be among the common bacteria found in the biological filters used in drinking water treatment, in drinking water, and biofilms (14, 15, 25, 26, 27, 30, 31, 36). In biological filters, the amount of monoenoic PLFAs typical for gram-negative bacteria has been 48 to 69% (25, 26, 27) and accounting for 25 to 39% in our biofilms and 26 to 52% in waters. Exceptionally, 16:1ω7c was detected only in minor amounts in biofilms, although it is one of the major fatty acids often found in PLFAs (48). Smith et al. (36) did not detect 16:1ω7c in water in water distribution system biofilms.
TABLE 1.
Percentages of PLFAs and LPS 3-OH-FAs and the ratio of C18 to C16 fatty acids in biofilms grown 4 and 11 weeks in nondisinfected drinking water with phosphate phosphorus (P) supplementation of 0, 1, 2, and 5 μg liter−1 and in warm water (45°C) and drinking water (7°C)
| FA type | Mean ± SE (n = 2)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biofilm (growth time, 4 wk) with P at:
|
Biofilm (growth time, 11 wk) with P at:
|
Warm water | Drinking water | |||||||
| 0 μg liter−1 | 1 μg liter−1 | 2 μg liter−1 | 5 μg liter−1 | 0 μg liter−1 | 1 μg liter−1 | 2 μg liter−1 | 5 μg liter−1 | |||
| PLFAs | ||||||||||
| i-14:0 | 0.18 ± 0.04 | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.11 ± 0.06 | 0.20 ± 0.06 | 0.14 ± 0.02 | 0.03 ± 0.01 | 0.12 ± 0.01 | 0.35 ± 0.03 | 0.67 ± 0.04 |
| 14:1 | ND | ND | ND | ND | ND | ND | ND | ND | 1.39 ± 0.46 | 2.27 ± 0.83 |
| 14:0 | 9.38 ± 0.55 | 5.83 ± 0.08 | 3.87 ± 1.19 | 5.81 ± 0.61 | 7.27 ± 3.15 | 3.52 ± 0.56 | 7.03 ± 1.34 | 1.51 ± 0.05 | 3.41 ± 1.14 | 4.35 ± 0.93 |
| br-15:0a | 1.01 ± 0.77 | 0.79 ± 0.57 | 2.10 ± 0.53 | 0.49 ± 0.33 | 1.07 ± 0.69 | TR | ND | 0.54 ± 0.30 | 0.17 ± 0.05 | 0.22 ± 0.03 |
| br-15:0b | 0.24 ± 0.05 | 0.96 ± 0.65 | 6.47 ± 0.52 | 1.25 ± 1.15 | TR | 5.23 ± 0.76 | 0.52 ± 0.43 | ND | 0.11 ± 0.12 | 0.48 ± 0.07 |
| i-15:0 | 0.51 ± 0.09 | 0.49 ± 0.13 | 0.40 ± 0.08 | 0.62 ± 0.42 | 1.45 ± 1.01 | 0.58 ± 0.09 | 0.15 ± 0.04 | 0.45 ± 0.07 | 1.82 ± 0.16 | 1.71 ± 0.29 |
| a-15:0 | 0.63 ± 0.09 | 0.49 ± 0.01 | 0.62 ± 0.10 | 0.44 ± 0.21 | 1.06 ± 0.44 | 0.53 ± 0.11 | 0.19 ± 0.03 | 0.41 ± 0.05 | 1.24 ± 0.01 | 1.88 ± 0.25 |
| 15:0 | 4.50 ± 0.75 | 2.27 ± 0.89 | 2.31 ± 0.08 | 1.35 ± 0.68 | 3.09 ± 1.24 | 1.60 ± 0.14 | 0.46 ± 0.14 | 0.37 ± 0.04 | 0.98 ± 0.13 | 0.33 ± 0.28 |
| i-16:0 | 0.47 ± 0.05 | 0.52 ± 0.03 | 0.67 ± 0.06 | 0.51 ± 0.29 | 1.13 ± 0.62 | 0.45 ± 0.06 | 0.26 ± 0.02 | 0.52 ± 0.11 | 0.96 ± 0.01 | 0.80 ± 0.04 |
| 16:1ω7c | 0.47 ± 0.11 | TR | 6.31 ± 4.24 | 4.91 ± 3.03 | 0.79 ± 0.07 | 1.84 ± 0.59 | 1.11 ± 0.84 | 4.45 ± 1.42 | 7.77 ± 0.61 | 27.96 ± 5.60 |
| 16:1ω5c | 0.78 ± 0.07 | TR | ND | ND | TR | ND | ND | ND | 0.70 ± 0.04 | TR |
| 16:0 | 29.41 ± 2.37 | 32.06 ± 0.48 | 28.58 ± 1.20 | 29.18 ± 1.13 | 29.80 ± 3.99 | 29.45 ± 0.72 | 28.84 ± 0.56 | 30.86 ± 0.60 | 32.10 ± 0.42 | 24.08 ± 2.44 |
| 10-Me-16:0 | 0.28 ± 0.02 | TR | 0.12 ± 0.06 | TR | 0.78 ± 0.13 | TR | 0.25 ± 0.13 | ND | 4.68 ± 1.37 | 1.07 ± 0.12 |
| i-17:0 | 0.47 ± 0.05 | 0.64 ± 0.20 | 0.42 ± 0.07 | 0.77 ± 0.36 | 1.88 ± 1.34 | TR | TR | 0.57 ± 0.04 | 0.58 ± 0.01 | 0.37 ± 0.05 |
| a-17:0 | 1.09 ± 0.12 | 1.12 ± 0.31 | 0.71 ± 0.14 | 0.82 ± 0.20 | 1.09 ± 0.32 | 0.74 ± 0.09 | 0.58 ± 0.09 | 0.78 ± 0.03 | 0.55 ± 0.03 | 0.43 ± 0.02 |
| cy-17:0 | 1.13 ± 0.20 | TR | 1.99 ± 1.03 | ND | 0.81 ± 0.05 | 1.16 ± 0.08 | TR | ND | 1.97 ± 0.34 | TR |
| i-18:0 | 0.42 ± 0.03 | TR | 0.34 ± 0.01 | 0.33 ± 0.09 | 0.32 ± 0.16 | 0.18 ± 0.02 | 0.25 ± 0.02 | 0.34 ± 0.03 | TR | TR |
| 18:2ω6c | 1.53 ± 0.31 | TR | ND | ND | 0.88 ± 0.18 | 2.55 ± 1.00 | TR | TR | ND | ND |
| 18:1ω9c | 8.06 ± 0.68 | 8.07 ± 0.04 | 4.38 ± 0.53 | 7.12 ± 0.93 | 9.86 ± 1.19 | 6.51 ± 1.78 | 8.25 ± 1.06 | 6.32 ± 0.84 | 3.44 ± 0.16 | 4.39 ± 0.40 |
| 18:1ω7c | 14.39 ± 0.20 | 18.89 ± 1.27 | 14.89 ± 2.28 | 17.63 ± 6.37 | 14.40 ± 1.05 | 15.60 ± 0.07 | 21.70 ± 1.53 | 26.95 ± 0.50 | 13.03 ± 2.29 | 16.96 ± 2.57 |
| 18:0 | 24.88 ± 0.81 | 25.37 ± 2.38 | 25.64 ± 6.82 | 28.54 ± 1.38 | 23.11 ± 0.58 | 28.53 ± 3.61 | 29.54 ± 2.59 | 24.79 ± 1.37 | 21.36 ± 0.47 | 10.87 ± 4.08 |
| TBSA | ND | ND | ND | ND | ND | ND | ND | ND | 0.59 ± 0.14 | ND |
| cy-19:0 | TR | ND | TR | TR | 0.60 ± 0.40 | 0.15 ± 0.08 | ND | ND | 2.49 ± 0.31 | 0.31 ± 0.24 |
| C18/C16 ratio | 1.56 ± 0.18 | 1.53 ± 0.21 | 1.36 ± 0.46 | 1.62 ± 0.45 | 1.57 ± 0.23 | 1.62 ± 0.13 | 2.00 ± 0.20 | 1.65 ± 0.12 | 0.93 ± 0.09 | 0.62 ± 0.05 |
| LPS 3-OH-FAs | ||||||||||
| 3-OH-10:0 | 8.25 ± 1.86 | 26.39 ± 1.59 | 34.66 ± 4.48 | 32.18 ± 1.26 | 33.81 ± 5.53 | 21.33 ± 5.85 | 36.39 ± 4.65 | 29.74 ± 0.73 | 17.82 ± 2.81 | 25.82 ± 2.10 |
| 3-OH-12:0 | 28.00 ± 0.81 | 12.92 ± 0.15 | 25.02 ± 0.26 | 27.15 ± 5.96 | 27.48 ± 2.21 | 27.78 ± 3.24 | 17.18 ± 0.82 | 11.10 ± 1.13 | 11.40 ± 0.51 | 11.21 ± 0.08 |
| 3-OH-14:0 | 63.75 ± 1.05 | 60.70 ± 1.44 | 40.32 ± 4.22 | 40.67 ± 7.22 | 38.71 ± 3.32 | 50.89 ± 2.61 | 46.43 ± 3.83 | 59.16 ± 0.40 | 70.78 ± 3.32 | 62.97 ± 2.03 |
ND, not detected; TR, trace amounts detected.
iso- and anteiso-branched fatty acids, reflecting gram-positive bacteria (29), accounted for 1.7 to 7.1% of the total PLFAs in our samples (Table 1). Their abundance has been between 2.0 and 11% in drinking water biofilters (25, 27) and ca. 7.6% in drinking water biofilms (36). The indicator of mycobacteria and other actinomycetes, tuberculostearic acid (TBSA) (3), was only found in warm water. Actinomycetes have earlier been occasionally detected in Finnish drinking water distribution systems in concentrations of 2 to 100 CFU liter−1, the species found being members of Mycobacterium avium complex or Mycobacterium fortuitum (12, 28, 45). The eucaryotic marker fatty acid, 18:2ω6c (20, 32), was mainly detected in biofilms without and with 1 μg of phosphorus liter−1 supplementation. Fungi and yeasts have been frequently detected in Finnish drinking water in amounts of <90 CFU liter−1 (14, 28, 50).
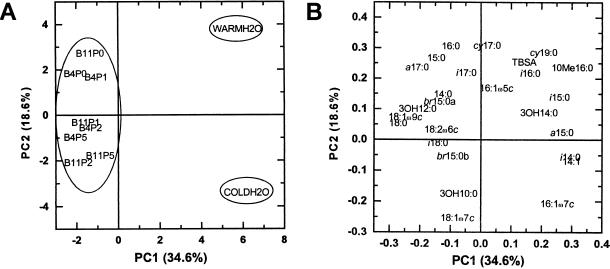
Principal component analyses separated the microbial community structure of drinking and warm water from that of biofilms (Fig. 1). The biofilms were positioned to the left and waters to the right along the PC1 axis, explaining 34.6% of the variation in results (Fig. 1A). The 14- to 16-carbon PLFAs i-14:0, i-15:0, i-16:0, a-15:0, 10-Me-16:0, 14:1, and 16:1ω7c and LPS 3-OH-14:0 were more characteristic of water samples than biofilms, and these acids were positioned to the right along the PC1 axis with positive loadings (Fig. 1B, Table 1). The PLFAs which were more characteristic of biofilms were the 17- to 18-carbon acids a-17:0, i-18:0, 18:1ω9c, and 18:2ω6c and also 14:0, 15:0, and 18:0, in addition to LPS 3-OH-10:0 and 3-OH-12:0, which were found to the left of the PC1 axis with negative loadings. Consequently, the ratio of C18 to C16 fatty acids was higher in warm water than in drinking water and highest in the biofilms.
FIG. 1.
(A) Score plot of PCA showing separation of drinking water (COLDH2O), warm water (WARMH2O), and biofilms along PC1 and PC2 (B4, biofilm growth time of 4 weeks; B11, biofilm growth time of 11 weeks; P0, phosphorus addition, 0 μg liter−1; P1, 1 μg liter−1; P2, 2 μg liter−1; and P5, 5 μg liter−1). Ellipses separate biofilms from warm water and drinking water. (B) Loading values for individual fatty acids along PC1 and PC2 axes.
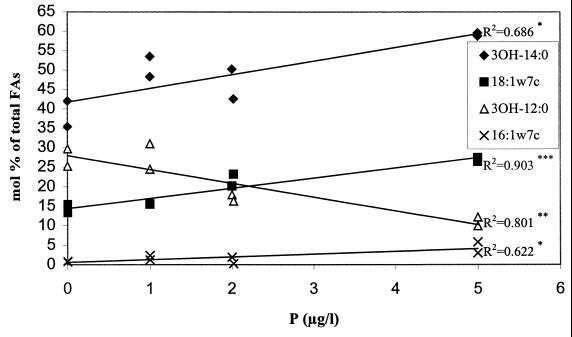
The biofilms grown without phosphate phoshorus supplementation or grown for 4 weeks with an addition of 1 μg of phosphorus liter−1 were separated from those grown for 11 weeks with a supplementation of 2 or 5 μg of phosphorus liter−1 along the PC2 axis, which accounted for 18.6% of the variance in data (Fig. 1A). After 11 weeks of biofilm development, the amount of added phosphate correlated positively with the proportions of 16:1ω7c (P < 0.05, R2 = 0.622), 18:1ω7c (P < 0.001, R2 = 0.903), and 3-OH-14:0 (P < 0.05, R2 = 0.686) and negatively with the proportion of 3-OH-12:0 (P < 0.01, R2 = 0.801) (Fig. 2; Table 1). Thus, in biofilms grown for 11 weeks the addition of phosphate seemed to increase the proportion of gram-negative bacteria due to the increase in 16:1ω7c and 18:1ω7c. Their community structure may also have been altered due to the increase in LPS 3-OH-14:0 and a decrease in 3-OH-12:0 with the increase in phosphate. Concomitantly, the amount of other microbes might have slightly decreased. Otherwise, rather similar biofilms were formed already after 4 weeks of growth, independently of the amount of phosphate in water, as evaluated from PLFA compositions. The biofilm formation has been rapid both on polyvinylchloride and steel surfaces exposed to the treated artificial groundwater (49) and on glass surfaces exposed to the treated groundwater (43). The second principal component also differentiated drinking water from warm water (Fig. 1A). The PLFAs 16:0, 18:0, cy-17:0, cy-19:0, 10-Me-16:0, TBSA, and 16:1ω5c and LPS 3-OH-14:0 were more common in warm than drinking water (Fig. 1B, Table 1). In contrast, more 14:1, 16:1ω7c, 18:1ω7c, 18:1ω9c, and i-14:0 and LPS 3-OH-10:0 fatty acids were present in the PLFAs of drinking water than of warm water.
FIG. 2.
Amounts of representative PLFAs and LPS 3-OH-FAs in biofilms grown for 11 weeks with 0 to 5 μg of phosphate phosphorus liter−1 supplementation. Asterisks represent significance of correlation (✽, P < 0.05; ✽✽, P < 0.01; ✽✽✽, P < 0.001).
The index of microbial biomass, the mean amount of PLFAs in biofilms, was 332 ± 62 ng cm−2 (1.18 ± 0.22 nmol cm−2), corresponding to (2.37 ± 0.45) × 107 cells cm−2, with no phosphate-dependent change in biomass. In contrast, in drinking water the phosphate addition has been shown to encourage microbial growth (4, 21, 23, 34, 35). However, in biofilms, which are very different environments from water, the effects of phosphate addition might be different. In river water biofilms the addition of 5 μM phosphate phosphorus increased the bacterial numbers maximally by approximately fivefold (24). The LPS 3-OH-FA content has been used to indicate the biomass of gram-negative bacteria (7, 51, 52) and was in biofilms on average 504 ± 43 pg cm−2 (2.13 ± 0.18 pmol cm−2) independent of the phosphate concentration. The amount of extracellular polymeric substances has been reduced with the increase in phosphate phosphorus in river water biofilms (24).
The sum of PLFAs was 540 ± 303 ng liter−1 (1.96 ± 0.78 nmol liter−1) in drinking water and 1.9-fold greater (1,050 ± 172 ng liter−1, 3.75 ± 0.43 nmol liter−1) in warm water. The LPS 3-OH-FA content in warm water (9.15 ± 0.79 ng liter−1, 37.42 ± 3.63 pmol liter−1) was 2.6 times the amount in drinking water (3.49 ± 1.24 ng liter−1, 14.48 ± 4.83 pmol liter−1). The calculated cell density was (3.92 ± 1.56) × 107 cells liter−1 in drinking water and (7.50 ± 0.87) × 107 cells liter−1 in warm water. According to the acridine orange staining the number of microbes has been shown to be only slightly higher in the warm than drinking water, whereas the cell volume was greater in warm water than in drinking water (50). The viable count in drinking water was 1.00 × 105 CFU liter−1. Thus, the number of microbes in the drinking water was very similar, estimated either from the PLFA content or R2A culture results, considering that <0.5% of all microbes are cultivable (22). The molar ratios of LPS 3-OH-FAs to PLFAs were 4.1 to 5.6 times smaller in biofilms than in waters, which might result from different environmental conditions. The microbes present in waters have survived the disinfection process and been retained in the water pipelines for 1 to 2 days, whereas biofilms were grown from drinking water directly after the water treatment without disinfection.
In conclusion, the addition of phosphate increased the proportion of 16:1ω7c and 18:1ω7c acids and affected LPS 3-OH-FAs, indicative of an increase in gram-negative bacteria and changes in their communities in biofilms grown for 11 weeks. However, the greatest differences in PLFAs were seen between waters and biofilms. The ratio of C18 to C16 acids was highest in biofilms (21°C), intermediate in warm water (45°C), and lowest in drinking water (7°C). The lower ratio of C18 to C16 fatty acids in free-living bacteria compared to adhered bacteria has been related with the heterogeneity in the culture, and the rapid selection of community by the surface to which they are attached (41). The differences between waters and biofilms might be due in part to different temperatures, since at low temperatures microbes may decrease the ratio of C18 to C16 acids and fatty acid cyclization and increase fatty acid unsaturation (37), all of which are differences observed between drinking water and warm water. However, the concentrations of mesophilic heterotrophic bacteria have been determined to be higher in drinking water than in warm water, whereas warm water favors the occurrence of moderate thermophilic heterotrophic bacteria (50). The differences in species of gram-negative bacteria between biofilms, warm water, and drinking water can be assumed from the differences in the LPS 3-OH-FA profiles.
Acknowledgments
We thank the staff on the Laboratory of Environmental Microbiology in the National Public Health Institute. We are also grateful to Ewen MacDonald for reading and commenting on the manuscript.
This study was supported by the Finnish Research Programme on Environmental Health (project 42676), the Academy of Finland (projects 34538 and 51999), and the Ministry of Agriculture and Forestry of Finland.
REFERENCES
- 1.Balkwill, D. E., F. R. Leach, J. T. Wilson, J. F. McNabb, and D. C. White. 1988. Equivalence of microbial biomass measures based on membrane lipid and cell wall components, adenosine triphosphate, and direct counts in subsurface aquifer sediments. Microb. Ecol. 16:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. [DOI] [PubMed] [Google Scholar]
- 3.Brennan, P. J. 1988. Mycobacterium and other actinomycetes, p.203–298. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 1. Academic Press, London, Great Britain.
- 4.Charnock, C., and O. Kjønnø. 2000. Assimilable organic carbon and biodegradable dissolved organic carbon in Norwegian raw and drinking water. Water Res. 34:2629–2642. [Google Scholar]
- 5.Emde, K. M. E., D. W. Smith, and R. Facey. 1992. Initial investigation of microbially influenced corrosion (mic) in a low temperature water distribution system. Water Res. 26:169–175. [Google Scholar]
- 6.Fass, S., M. L. Dincher, D. J. Reasoner, D. Gatel, and J.-C. Block. 1996. Fate of Escherichia coli experimentally injected in a drinking water distribution pilot system. Water Res. 30:2215–2221. [Google Scholar]
- 7.Federle, T. W., M. A. Hullar, R. J. Livingston, D. A. Meeter, and D. C. White. 1983. Spatial distribution of biochemical parameters, indicating biomass and community composition of microbial assemblies in estuarine mud flat sediments. Appl. Environ. Microbiol. 45:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnish Standards Association. 1986. Determination of total phosphorus in water digestion with peroxide sulfate, SFS 2026. Finnish Standards Association, Helsinki, Finland.
- 9.Frostegård, Å., A. Tunlid, and E. BåÅth. 1991. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 14:151–163. [Google Scholar]
- 10.Gradowska, W., and L. Larsson. 1994. Determination of absolute configurations of 2- and 3-hydroxy fatty acids in organic dust by gas chromatography-mass spectrometry. J. Microbiol. Methods 20:55–67. [Google Scholar]
- 11.Jantzen, E., T. Tangen, and J. Eng. 1989. Gas chromatography of mycobacterial fatty acids and alcohols: diagnostic applications. APMIS 97:1037–1045. [DOI] [PubMed] [Google Scholar]
- 12.Kauppinen, J., T. Nousiainen, E. Jantunen, R. Mattila, and M.-L. Katila. 1999. Hospital water supply as a source of disseminated Mycobacterium fortuitum infection in a leukemia patient. Infect. Control Hosp. Epidemiol. 20:343–345. [DOI] [PubMed] [Google Scholar]
- 13.King, J. D., D. C. White, and C. W. Taylor. 1977. Use of lipid composition and metabolism to examine structure and activity of estuarine detrial microflora. Appl. Environ. Microbiol. 33:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahti, K. 1993. Microbial quality of drinking water in some Finnish distribution systems. Water Sci. Technol. 27:151–154. [Google Scholar]
- 15.LeChevallier, M. W., T. M. Babcock, and R. G. Lee. 1987. Examination and characterization of distribution systems biofilms. Appl. Environ. Microbiol. 53:2714–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Factors promoting survival of bacteria in chlorinated water supplies. Appl. Environ. Microbiol. 54:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Inactivation of biofilm bacteria. Appl. Environ. Microbiol. 54:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeChevallier, M. W., W. Schulz, and R. G. Lee. 1991. Bacterial nutrients in drinking water. Appl. Environ. Microbiol. 57:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehtola, M. J., I. T. Miettinen, T. Vartiainen, and P. J. Martikainen. 1999. A new sensitive bioassay for determination of microbially available phosphorus in water. Appl. Environ. Microbiol. 65:2032–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lösel, D. M. 1988. Fungal lipids, p.699–806. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 1. Academic Press, London, Great Britain.
- 21.Miettinen, I. T., T. Vartiainen, and P. J. Martikainen. 1996. Contamination of drinking water. Nature 381:654–655. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen, I. T., T. Vartiainen, and P. J. Martikainen. 1997. Changes in water microbial quality during bank filtration of lake water. Can. J. Microbiol. 43:1126–1132. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen, I. T., T. Vartiainen, and P. J. Martikainen. 1997. Phosphorus and bacterial growth in drinking water. Appl. Environ. Microbiol. 63:3242–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed, M. N., J. R. Lawrence, and R. D. Robarts. 1998. Phosphorus limitation of heterotrophic biofilms from the Fraser river, British Columbia, and the effect of pulp mill effluent. Microb. Ecol. 36:121–130. [DOI] [PubMed] [Google Scholar]
- 25.Moll, D. M., and R. S. Summers. 1999. Assessment of drinking water filter microbial communities using taxonomic and metabolic profiles. Water Sci. Technol. 39:83–89. [Google Scholar]
- 26.Moll, D. M., R. S. Summers, and A. Breen. 1998. Microbial characterization of biological filters used for drinking water treatment. Appl. Environ. Microbiol. 64:2755–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moll, D. M., R. S. Summers, A. C. Fonseca, and W. Matheis. 1999. Impact of temperature on drinking water biofilter performance and microbial community structure. Environ. Sci. Technol. 33:2377–2382. [Google Scholar]
- 28.Niemi, R. M., S. Knuth, and K. Lundström. 1982. Actinomycetes and fungi in surface waters and in potable water. Appl. Environ. Microbiol. 43:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary, W. M., and S. G. Wilkinson. 1988. Gram-positive bacteria, p.117–201. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 1. Academic Press, London, Great Britain.
- 30.Payment, P., F. Gamache, and G. Paquette. 1988. Microbial and virological analysis of water from two water filtration plants and their distribution systems. Can. J. Microbiol. 34:1304–1309. [DOI] [PubMed] [Google Scholar]
- 31.Percival, S. L., J. S. Knapp, R. Edyvean, and D. S. Wales. 1998. Biofilm development on stainless steel in mains water. Water Res. 32:243–253. [Google Scholar]
- 32.Rattray, J. B. M. 1988. Yeasts, p.555–697. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 1. Academic Press, London, Great Britain.
- 33.Reasoner, D. J., and E. E. Geldreich. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sathasivan, A., S. Ohgaki, K. Yamamoto, and N. Kamiko. 1997. Role of inorganic phosphorus in controlling regrowth in water distribution systems. Water Sci. Technol. 35:37–44. [Google Scholar]
- 35.Sathasivan, A., and S. Ohgaki. 1999. Application of new bacterial regrowth potential method for water distribution systems: a clear evidence of phosphorus limitation. Water Res. 33:137–144. [Google Scholar]
- 36.Smith, C. A., C. B. Phiefer, S. J. MacNaughton, A. Peacock, R. S. Burkhalter, R. Kirkegaard, and D. C. White. 2000. Quantitative lipid biomarker detection of unculturable microbes and chlorine exposure in water distribution system biofims. Water Res. 34:2683–2688. [Google Scholar]
- 37.Suutari, M., and S. Laakso. 1994. Microbial fatty acids and thermal adaptation. Crit. Rev. Microbiol. 20:285–328. [DOI] [PubMed] [Google Scholar]
- 38.Suutari, M., K. Liukkonen, and S. Laakso. 1990. Temperature adaptation in yeasts: the role of fatty acids. J. Gen. Microbiol. 136:1469–1474. [DOI] [PubMed] [Google Scholar]
- 39.Torkko, P., M. Suutari, S. Suomalainen, L. Paulin, L. Larsson, and M.-L. Katila. 1998. Separation among species of Mycobacterium terrae complex by lipid analyses: comparison with biochemical tests and 16S rRNA sequencing. J. Clin. Microbiol. 36:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunlid, A., D. Ringelberg, T. J. Phelps, C. Low, and D. C. White. 1989. Measurement of phospholipid fatty acids at picomolar concentrations in biofilms and deep subsurface sediments using gas chromatography and chemical ionization mass spectrometry. J. Microbiol. Methods 10:139–152. [Google Scholar]
- 41.Valeur, A., A. Tunlid, and G. Odham. 1988. Differences in lipid composition between free-living and initially adhered cells of gram-negative bacterium. Arch. Microbiol. 149:521–526. [Google Scholar]
- 42.van der Kooij, D. 1992. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Works Assoc. 84:57–65. [Google Scholar]
- 43.van der Kooij, D., H. R. Veenendaal, C. Baars-Lorist, D. W. van der Klift, and Y. C. Drost.1995. Biofilm formation on surfaces of glass and Teflon exposed to treated water. Water Res. 29:1655–1662. [Google Scholar]
- 44.van der Wende, E., W. G. Characklis, and D. B. Smith. 1989. Biofilms and bacterial drinking water quality. Water Res. 23:1313–1322. [Google Scholar]
- 45.von Reyn, C. F., R. D. Waddell, T. Eaton. R. D. Arbeit, J. N. Maslow, T. W. Barber, R. J. Brindle, C. F. Gilks, J. Lumio, J. A. Ranki, D. Dawson, and J. O. Falkinham III. 1993. Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and Kenya. J. Clin. Microbiol. 31:3227–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward, D., M. R. Weller, and M. M. Bateson. 1990. 16S rRNA sequences reveal numerous uncultured inhabitants in natural community. Nature 345:63–65. [DOI] [PubMed] [Google Scholar]
- 47.White, D. C., W. M. Davis, J. S. Nickels, J. D. King, and R. J. Bobbie. 1979. Determination of sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson, S. G. 1988. Gram-negative bacteria, p.299–488. In C. Ratledge and S. G. Wilkinson (ed.), Microbial lipids, vol. 1. Academic Press, London, Great Britain.
- 49.Zacheus, O. M., E. K. Iivanainen, T. K. Nissinen, M. J. Lehtola, and P. J. Martikainen. 2000. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 34:63–70. [Google Scholar]
- 50.Zacheus, O. M., and P. J. Martikainen. 1995. Occurrence of heterotrophic bacteria and fungi in cold and warm water distribution systems using water of different quality. Can. J. Microbiol. 41:1088–1094. [Google Scholar]
- 51.Zelles, L., Q. Y. Bai, T. Beck, and F. Beese. 1992. Signature fatty acids in phospholipid and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soil. Soil Biol. Biochem. 24:317–323. [Google Scholar]
- 52.Zelles, L., Q. Y. Bai, R. Rackwitz, D. Chadwick, and F. Beese. 1995. Determination of phospholipid- and lipopolysaccharide-derived fatty acids as an estimate of microbial biomass and community structures in soils. Biol. Fertil. Soils 19:115–123. [Google Scholar]