Abstract
The thermophilic cyanobacterium Synechococcus sp. strain MA19 contained the structural genes for cyanophycin synthetase (cphA) and cyanophycinase (cphB), which were identified, cloned, and sequenced in this study. The translation products of cphA and cphB exhibited high levels of similarity to corresponding proteins of other cyanobacteria, such as Anabaena variabilis and Synechocystis sp. Recombinant cells of Escherichia coli harboring cphA colinear with lacPO accumulated cyanophycin that accounted for up to 25% (wt/wt) of the dry cell matter in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG). The cyanophycin synthetase was enriched 123-fold to electrophoretic homogeneity from the soluble fraction of the recombinant cells by anion-exchange chromatography, affinity chromatography, and gel filtration chromatography. The purified cyanophycin synthetase maintained the parental thermophilic character and was active even after prolonged incubation at 50°C; in the presence of ectoine the enzyme retained 90% of its activity even after 2 h of incubation. The in vitro activity of the enzyme depended on ATP, primers, and both substrates, l-arginine and l-aspartic acid. In addition to native cyanophycin, the purified enzyme accepted a modified cyanophycin containing less arginine, α-arginyl aspartic acid dipeptide, and poly-α,β-dl-aspartic acid as primers and also incorporated β-hydroxyaspartic acid instead of l-aspartic acid or l-canavanine instead of l-arginine at a significant rate. The lack of specificity of this thermostable enzyme with respect to primers and substrates, the thermal stability of the enzyme, and the finding that the enzyme is suitable for in vitro production of cyanophycin make it an interesting candidate for biotechnological processes.
Cyanophycin is a reserve material for nitrogen and energy found only in cyanobacteria and was first observed by Borzi in 1887 (5). Accumulation of cyanophycin normally occurs when the cells enter the stationary growth phase (2, 17, 19, 28), in cells grown under stress conditions (14, 15), or in some specific cell forms, such as akinetes and heterocysts (10). Synthesis of cyanophycin occurs via a nonribosomal pathway catalyzed by cyanophycin synthetase and can be stimulated by adding antibiotics, such as chloramphenicol, to growing cultures (29) or by reducing the photon flow and cultivation temperature (38). Cyanophycin granules are not the primary nitrogen source for de novo synthesis of protein. However, they are mobilized during akinete germination (35). Cyanophycin may also serve as an energy source. Under strictly anaerobic conditions ATP is formed during ornithine formation from arginine (33). Cyanophycin contents depend on the species and range from 0.1 to 16% (wt/wt) of the dry cell matter. Cyanophycin is a branched polypeptide that normally consists of l-aspartic acid and l-arginine at a molar ratio of approximately 1:1 and is therefore also referred to as multi-l-arginine-poly-l-aspartic acid (30, 32, 34). The polymer is of biotechnological interest since if the arginine content is reduced (11), the oligo-arginyl polyaspartic acid obtained may be used as a biodegradable substitute in various technical products and processes (27).
Cyanophycin synthetases have been enriched 94-fold from Anabaena cylindrica (31) and 144-fold from the thermostable cyanobacterium Synechococcus sp. strain MA19 (8), and a cyanophycin synthetase was also purified to electrophoretic homogeneity from Anabaena variabilis (39). In Synechocystis sp. strain PCC6803 the enzyme is encoded by cphA (39), which is represented by slr2002 in the genomic library of this strain (12). The cphA genes from A. variabilis, Synechocystis sp. strain PCC6803, and Synechocystis sp. strain PCC6308 have been cloned and expressed in Escherichia coli (1, 22, 39). Previously, cphA genes have been described for six different strains of cyanobacteria. These strains are members of species of the genera Anabaena, Synechococcus, Synechocystis, and Cyanothece (1, 3, 22, 39; GenBank accession no. AAF97933). Amino acid sequence analysis revealed high levels of similarity to the ATP-dependent carboxylate amine/thiol ligases belonging to the superfamily of substrate ligases involved in biosynthesis of bacterial peptidoglycan (murein ligases) and to the capB translation product responsible for poly-γ-d-glutamate synthetase in Bacillus anthracis (7, 18, 36). The enzyme cyanophycin synthetase contains two ATP and magnesium binding sites and four other conserved regions (1, 3).
cphB, which encodes the intracellular cyanophycinase, was cloned from Synechocystis sp. strain PCC6803 and expressed in E. coli (23). This serine type of endopeptidase was capable of hydrolyzing cyanophycin in vitro into arginyl aspartic acid dipeptides (23). cphB and cphA are in the same cluster and are oriented colinearly but do not seem to be cotranscribed (39).
In this paper we describe cloning of cphA and cphB from the thermophilic organism Synechococcus sp. strain MA19. Biochemical characterization of the thermostable cyanophycin synthetase provided information which could be useful for in vitro synthesis of cyanophycin on a technical scale.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
An axenic culture of Synechococcus sp. strain MA19 (20), which was also used in a previous study (8), was a gift from the culture collection of the Molecular Bioenergetics Laboratory of the National Institute of Bioscience and Human Technology (Tsukuba, Ibaraki, Japan). It was grown photoautotrophically at 50°C in liquid BG11 medium (24) with irradiation (100 microeinsteins s−1 m−2) and shaking (100 rpm). Growth of the cyanobacterium was started with a short chromatic adaptation period (10 h of irradiation at 50 microeinsteins s−1 m−2). The E. coli strains were grown at 37°C in Luria-Bertani medium (26) with shaking (150 rpm). For cultivation of plasmid-carrying E. coli, 75 μg of ampicillin per ml was added. The bacterial strains, plasmids, and DNA fragments used in this study are listed in Table 1. Fresh competent cells of E. coli were prepared by using the CaCl2 standard method (26).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or phenotype | Source |
|---|---|---|
| Synechococcus sp. strain MA 19 | Thermophilic, optimal growth at 50°C | NIBHTa |
| Escherichia coli strains | ||
| TOP10 | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 ΔlacU169 (φ80 lacZΔM15) | Invitrogen (San Diego, Calif.) |
| XL1 Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lac1qZΔM15Tn10(Tetr)] | Stratagene |
| Plasmids | ||
| pBluescript SK(−) | Amprlac POZ′, T7 and T3 promotor | Stratagene |
| pSK::H6.5 | 6.5-kbp HindIII fragment from Synechococcus sp. strain MA19 genomic DNA harboring complete cphB and 5′ part of cphA inserted in pBluescript SK(−) | This study |
| pSK::Kp1.7 | 1.7-kbp inverse PCR product from Synechococcus sp. strain MA19 genomic DNA containing 3′ region of cphA inserted in pBluescript SK(−) | This study |
| pSK::cphAMA19co | 2.98-kbp PCR product from Synechococcus sp. strain MA19 genomic DNA carrying cphA inserted in pBluescript SK(−) colinear with respect to lacPO | This study |
| pSK::cphAMA19anti | 2.98-kbp PCR product from Synechococcus sp. strain MA19 genomic DNA carrying cphA inserted in pBluescript SK(−) antilinear with respect to lacPO | This study |
NIBHT, National Institute of Bioscience and Human Technology, Tsukuba, Ibaraki, Japan.
Isolation of cyanophycin.
Cyanophycin was isolated from recombinant cells of E. coli harboring the cphA gene of Synechococcus sp. MA19 by taking advantage of its solubility properties and using the method described by Simon (31), with two modifications (0.4% [vol/vol] Triton X-100 was added to the extraction buffer, and the sonicated cells were treated with one cycle of heating and cooling [heating at 95°C for 5 min followed by cooling at 0°C for 5 min]). The purity of the cyanophycin isolated and its amino acid composition were analyzed by sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) and by reversed-phase high-performance liquid chromatography (RP18 column; Techsphere ODS-2; Kontron Instruments GmbH, Neufahrn, Germany) after precolumn derivatization with phthaldialdehyde reagent (Merck, Hohenbrunn, Germany) as described recently (8).
Electrophoretic methods.
For SDS-polyacrylamide gel electrophoresis, 11.5% (wt/vol) polyacrylamide gels were prepared as described by Laemmli (13). Molecular weight marker proteins were purchased from Bio-Rad. Proteins and cyanophycin were stained with Serva Blue R. For protein determinations we used the method of Bradford (6). For analysis of nucleic acids, electrophoresis in 0.8 and 1.1% (wt/vol) agarose gels was performed by standard methods (26).
DNA manipulation and construction of a partial genomic library.
Total genomic DNA of Synechococcus sp. strain MA19 was extracted by a modified method described recently (9). Protein impurities were removed by using a large amount of proteinase K (1 mg ml−1). Plasmid DNA was isolated from E. coli by the alkaline extraction method (4). Purified DNA was digested with restriction enzymes according to the instructions of the manufacturer (Gibco, BRL). Southern hybridization was carried out by standard methods. A 1.87-kbp DNA fragment harboring part of cphA from Nostoc ellipsosporum NE1 was labeled by using a DIG-High Prime kit (Boehringer, Mannheim, Germany). HindIII DNA restriction fragments that were 5 to 7 kbp long were cut from a 0.8% (wt/vol) agarose gel and purified by using a NucleoTrap kit (Machery and Nagel, Düren, Germany). About 400 ng of the fragments was ligated to 400 ng of HindIII-digested pBluescript SK(−) DNA with T4 DNA ligase at 14°C overnight. The ligation products were transferred into E. coli XL1 Blue by using standard methods (26).
Screening of genomic libraries.
Genomic libraries were screened by colony hybridization at 53°C by using standard procedures (26). To visualize the chemoluminescent substrate chloro-5-substituted adamantyl 1,2-dioxetane phosphate (CSPD), a kit (Boehringer) was used. Transformants that gave the strongest signals were analyzed further. One positive clone, which carried a 6.5-kbp inserted fragment, was chosen for sequencing.
Inverse PCR to obtain the 3′ region of cphA.
Since cphA of Synechococcus sp. strain MA19 contained a HindIII restriction site in the 3′ region, an inverse PCR was performed as follows. Genomic DNA was digested with KpnI. About 150 ng of the purified KpnI restriction fragments was religated with T4 DNA ligase incubation for 6 h at 14°C. The circular DNA molecules formed were used for an inverse PCR performed with primer P1 (5′-AGAAAACAGCTCGGCTACACACTTT-3′) and primer P2 (5′-TTTAAGACGAAACACAAGCGATTAA-3′), whose sequences were deduced from the 3′-terminal region of the sequenced region of cphA from the positive clone with the 6.5-kbp HindIII fragment inserted. The primers were purchased from MWG-Biotech AG (Ebersberg, Germany). The PCR were performed by using Vent DNA polymerase (New England Biolabs, Schwalbach/Taunus, Germany) according to the instructions in the PCR applications manual (Biochemica 1995; Boehringer), and the 1.7-kbp product was purified with a Nucleotrap kit by following the instructions of the manufacturer (Machery and Nagel).
DNA sequencing and sequence analysis.
DNA sequences were determined by using a model 4000L DNA sequencer (LI-COR Inc., Biotechnology Division, Lincoln, Nebr.) and a Thermo Sequenase fluorescence-labeled primer cycle sequencing kit (Amersham Life Science, Buckinghamshire, United Kingdom) according to the instructions of the manufacturers. The nucleic acid sequences obtained from both strands were analyzed with a computer program from Heidelberg Unix Sequence Analysis Resources (HUSAR, release 4.0.; based on the Wisconsin Genetics Computer Group program package, version Unix-8.1, 1995). Sequences were compared and aligned by using the network service programs BLAST provided by the National Center for Biotechnology Information and ClustalW provided by the European Bioinformatics Institute at EMBL. The alignments used for construction of a phylogenetic tree were obtained by using the Genedoc and TreeView service programs (25) (http://www.psc.edu/biomed/genedoc or http://taxonomy.zoology.gla.ac.uk).
Cloning of cphA from Synechococcus sp. strain MA19.
The cphA gene was amplified from the genomic DNA by PCR as described above. The following primers were used for this reaction and were deduced from nucleotide sequences upstream and downstream of cphA (except for the nucleotides in italics, which were inserted to generate an NdeI restriction site plus a histidine-encoding triplet and a BamHI restriction site, respectively): primers cphAMAsense (5′-GTGTTTCCCATATGCATCACCATCACCATCACATGACACTGATTTGACCGCTA-3′) and cphAMAanti (5′-AAATTCCAGTGAGGATCCAGTCC-3′). For PCR we used a temperature program consisting of one denaturation cycle (95°C for 2 min) and 32 amplification cycles (95°C for 30 s, 56°C for 30 s, and 72°C for 2 min). For transformation, about 400 ng of the purified PCR product was ligated to 400 ng of EcoRV-linearized pBluescript SK(−) DNA. Transformation was performed with competent cells of E. coli TOP10 obtained by the CaCl2 method (26). Recombinant plasmids harboring cphA with a colinear orientation (pSK::cphAMA19co) and an antilinear orientation (pSK::cphAMA19anti) with respect to lacPO were obtained.
Purification of cyanophycin synthetase.
Cells of E. coli TOP10 harboring pSK−::cphAMA19co were cultivated as described above until the optical density at 578 nm of the cell suspension was 1.0 to 1.1. Then, 1.0 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture. After the stationary phase was reached, the cells were spun down (4,000 × g, 10 min, 4°C) and were washed once with extraction buffer containing 50 mM Tris-HCl (pH 8.2), 20 mM KCl, 1 mM MgCl2, 1 mM EDTA, and 5 mM 2-mercaptoethanol. The washed cells (approximately 30 g [wet weight]) were dissolved in 100 ml of the same buffer and were disintegrated by using a Sonoplus GM200 Sonifier (Bandelin Electronic, Berlin, Germany). The cell debris was removed by centrifugation (30,000 × g, 4°C, 15 min). The soluble protein fraction was obtained as supernatant after a second centrifugation (100,000 × g, 4°C, 1 h).
Enzyme purification was carried out by a three-step procedure, which was described recently (8). All steps were carried out at 4 to 7°C. A buffer composed of 50 mM Tris-HCl (pH 8.2), 20 mM KCl, 5 mM β-mercaptoethanol, and 1 mM EDTA was used throughout the purification procedure. The soluble protein fraction was dialyzed against this buffer and was applied to a Q Sepharose Hiload column (2.6 by 10 cm; bed volume, 53 ml; Amersham Pharmacia Biotech, Freiburg, Germany) equilibrated with the buffer. After the protein was washed with 100 ml of buffer, it was eluted with a KCl gradient (0 to 600 mM in 750 ml) at a flow rate of 2.5 ml min−1. Fractions containing high enzyme activity were combined, desalted, and concentrated by ultrafiltration with a YM 30 membrane (Amicon Corp., Lexington, Ky.). The concentrated preparation was applied to a triazine dye Procion Blue HE-RD Sepharose CL-4B column. The combined fractions exhibiting high activity were concentrated and applied to a Superdex 200 HR column (2.6 by 60 cm; bed volume, 330 ml; Amersham Pharmacia Biotech) equilibrated with buffer which contained 150 mM KCl. Proteins were eluted at a constant flow rate of 1.0 ml min−1. The most active fractions were desalted and concentrated to a concentration of 0.2 mg of protein ml−1.
Cyanophycin synthetase assay.
For the cyanophycin synthetase assay we used a previously described protocol (31) modified as described recently (1). To simplify the calculations and minimize the radioactive waste, a reaction mixture containing 50 mM Tris (pH 8.2), 20 mM MgCl2, 20 mM KCl, 10 mM dithiothreitol, 3 mM ATP, 5 mM l-aspartic acid, 0.495 mM l-arginine, 5 μM radiolabeled l-[14C]arginine (Amersham Pharmacia Biotech), and enzyme solution (4 to 50 μg of protein) was used. The reaction mixture was overlaid with 30 μl of wax to prevent evaporation. The enzyme reaction was carried out at 30°C for 15 min and was stopped by diluting the mixture with 10 volumes of ice-cold water. The labeled products were collected by centrifugation (14,000 × g, room temperature, 20 min). The final steps were performed by using the method described previously (1). Scintillation counting was carried out with a model LS 6500 scintillation counter (Beckman Instruments GmbH, Munich, Germany).
Primers and chemicals used for in vitro synthesis of cyanophycin.
The primers and chemicals used for in vitro synthesis of cyanophycin were purchased from Sigma (α-arginyl aspartic acid dipeptide, lots 197FO334 and 125HO515); Calbiochem (β-hydroxyaspartic acid, lot 511235); Acros Organics (N-acetylglucosamine, 2-acetamido-2-deoxy-alpha-d-glucopyranose, lot A009910201); Bayer, Leverkusen, Germany (chemosynthetic poly-α,β-dl-aspartic acid LPOC618); and Biotop Gesellschaft für biotechnische Optimierung mbH, Witten, Germany [ectoine, (S)-2-methyl-1,4,5,6-tetrahydroxypyrimidine-4-carboxylic acid (purity, >97%)]. Aspartic acid-rich biopolymer was isolated from capsules of a Klebsiella sp. isolate which was capable of forming slimy colonies on agar mineral medium, by precipitation in 80% (vol/vol) ethanol. Cyanophycin-free cell membranes of Synechococcus sp. strain PCC7942, which probably contains no cyanophycin synthetase (39), were obtained by triplicate extraction of a cell debris fraction with 0.1 N HCl. Since this strain contained no cyanophycin (data not shown), the preparation obtained was considered a cyanophycin-free cyanobacterial membrane particle preparation. Modified cyanophycin was obtained by hydrolyzing 10 mg of cyanophycin in 30 mM acetic acid at 105°C for 4 h. In this way arginine was partially removed from the polymer chain (30). Cyanophycin with a reduced arginine content was purified by titration with NaOH and was washed with cold distilled water. The modified cyanophycin consisted of aspartic acid and arginine at a molar ratio of 1:0.6, which was determined by amino acid analysis by high-performance liquid chromatography with precolumn derivatization as described recently (8).
Nucleotide sequence accession number.
The nucleotide and amino acid sequence data determined in this study have been deposited in the GenBank database under accession no. AF329282.
RESULTS
Cloning of cphA and cphB.
In order to identify and clone the cyanophycin biosynthesis genes from the thermophilic organism Synechococcus sp. strain MA19, we used a strategy for construction of a partial genomic library based on Southern hybridization with HindIII-digested MA19 DNA and a labeled DNA probe harboring cphA of N. ellipsosporum NE1 (see Materials and Methods) (unpublished data). Fragments ranging from 5 to 7 kbp long from HindIII-digested genomic DNA were ligated to HindIII-linearized pBluescript SK(−) DNA and transformed into E. coli XL1 Blue. Approximately 1,000 transformants were selected and used for colony hybridization with the heterologous DNA probe, and 24 of these transformants produced signals which revealed that they harbored a 6.5-kbp DNA fragment insertion.
DNA sequencing of the 6.5-kbp HindIII insert revealed a 4.193-kbp region harboring two colinear open reading frames, ORF1 and ORF2. ORF1 consisted of 834 bp, and its translation product consisted of 277 amino acids. As the translation product exhibited levels of similarity of 86, 69, 63, and 57% to the cphB translation products of A. variabilis (39), Synechocystis sp. strain PCC6308 (1), Synechocystis sp. strain PCC6803 (22, 39), and Synechococcus elongatus (3), respectively, ORF1 was identified as cphB encoding a cyanophycinase. In ORF2, which was located 174 bp downstream from and colinear with cphB, the 3′ region was not present on the 6.5-kbp HindIII fragment. In order to clone this missing region, inverse PCR (37) was employed. Total genomic DNA was completely digested with KpnI, for which a corresponding restriction site was localized in the previously sequenced region of ORF2, and ligated to obtain circular DNA molecules, which were then employed as templates for PCR performed with primers P1 and P2 (see Materials and Methods). The 1.7-kbp PCR product included the 3′ region of ORF2. ORF2 was then completely sequenced. ORF2 consisted of 2.712 kbp, which encoded 903 deduced amino acids. Alignment of the CphA proteins from A. variabilis ATCC 29413 (= strain FD), S. elongatus, Synechocystis sp. strain PCC6803, Cyanothece sp. strain ATCC 51142, and Synechocystis sp. strain PCC6308 and the ORF2 translation product revealed high levels of similarity, especially in the amino-terminal regions (Fig. 1). The amino acid sequence deduced from cphA (903 amino acids) also exhibited striking levels of similarity in almost complete overlaps with the cyanophycin synthetases encoded by the cphA genes of A. variabilis ATCC 29413 (= strain FD), S. elongatus, Synechocystis sp. strain PCC6803, Cyanothece sp. strain ATCC 51142, and Synechocystis sp. strain PCC6308 (85, 67, 67, 68, and 64%, respectively). In addition, these sequences exhibited high levels of similarity with the ATP-grasp fold-containing glutathione synthetase and the murein and folyl poly-γ-d-glutamate ligase superfamilies of E. coli (7, 36). Region V in the carboxy-terminal amino acid sequences of CphA contained characteristic aspartic acid-rich stretches (Fig. 1).
FIG. 1.
Similarities among amino acid sequences deduced from the sequences of six cphA genes. The sequences of A. variabilis ATCC 29173 (GenBank accession no. AAF97933) (line i), Synechocystis sp. strain PCC6803 (12) (line ii), Synechocystis sp. strain PCC6308 (1) (line iii), Cyanothece sp. strain ATCC 51142 (GenBank accession no. AAF97933) (line iv), S. elongatus (3) (line v), and Synechococcus sp. strain MA19 (this study) (line vi) were compared. The consensus sequence is shown on line vii. The amino acids are indicated by standard one-letter abbreviations. Amino acid residues which are identical to the residues in the gene product of cphAMA19 are highlighted. The B-loop and J-loop regions corresponding to the ATP- and Mg2+-binding sites of cphA (3) and five other important regions are indicated by arrows and/or are underlined.
Heterologous expression of cphA and characterization of the enzyme products.
In order to determine whether Synechococcus sp. strain MA19 cphA is functionally active, expression of this gene in E. coli was examined. To do this, the cphA-carrying region was amplified from genomic DNA by using primers cphAMAsense and cphAMAanti. The 2.98-kbp PCR product, containing cphA plus the intergenic region between cphB and cphA, was ligated into EcoRV-restricted pBluescript SK(−). Recombinant strains of E. coli harboring cphA either in a colinear orientation (pSK::cphAMA19co) or in an antilinear orientation (pSK::cphAMA19anti) with respect to lacPO were analyzed for heterologous expression. Strong expression of cphA occurred in cells harboring pSK::cphAMA19co in the presence of IPTG. No activity was detected when the gene was transcribed from pSK::cphAMA19anti (Table 2). Polymeric material that accounted for up to 25% (wt/wt) of the cell dry matter was isolated from cells harboring pSK::cphAMA19co. The polymeric material was composed of aspartic acid and arginine at a molar ratio 1:0.98, which is typical for cyanophycin molecules. The electrophoretic mobility of this polymer is shown in Fig. 2b (lane 1).
TABLE 2.
Expression of Synechococcus sp. strain MA19 cphA and accumulation of cyanophycin in recombinant E. colia
| Plasmid | Orientationb | IPTGc | Cyanophycin content (% [wt/wt] of cell dry matter) | Sp actd |
|---|---|---|---|---|
| pSK::cphAMA19co | Colinear | + | 25.0 ± 2.0 | 1.81 ± 0.1 |
| − | 11.5 ± 2.0 | 0.66 ± 0.1 | ||
| pSK::cphAMA19anti | Antilinear | + | 0.5 ± 0.2 | 0.05 ± 0.1 |
| − | 0.5 ± 0.1 | 0.05 ± 0.1 | ||
| pBluescript SK(−) | Not relevant | + | 0.1 ± 0.1 | 0.05 ± 0.1 |
The experiments and enzyme assays were done in triplicate, and the standard errors were calculated by using standard statistics (P < 0.05).
Orientation with respect to lacPO.
Recombinant E. coli TOP10 cells were grown in Luria-Bertani medium in the presence (+) or in the absence (−) of 1.0 mM IPTG as described in Materials and Methods.
Specific activity is expressed as the rate of incorporation of l-arginine in nanomoles per minute per milligram of protein.
FIG. 2.
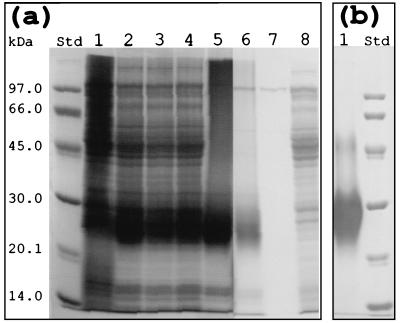
SDS-PAGE analysis of the in vitro-synthesized cyanophycin-like polymers. (a) Reactions catalyzed by the cyanophycin synthetase-containing soluble cell fraction (0.5 mg of protein ml−1) and by the purified cyanophycin synthetase (0.04 mg of protein ml−1) at 30°C overnight in the presence of different primers at a concentration of 1 mg ml−1 as described in Materials and Methods. Lane 1, soluble cell fraction with cyanophycin purified from Synechococcus sp. strain MA19; lane 2, soluble cell fraction with chemosynthetic poly-α,β-dl-aspartic acid; lane 3, soluble cell fraction with N-acetylglucosamine; lane 4, soluble cell fraction with α-arginyl aspartic acid dipeptide (lot 197FO334); lane 5, cyanophycin purified from Synechococcus sp. strain MA19; lane 6, purified cyanophycin synthetase with the α-arginyl aspartic acid dipeptide (lot 197FO334); lane 7, purified cyanophycin synthetase; lane 8, soluble cell fraction. (b) Lane 1, cyanophycin purified from E. coli (cphAMA19co). The sizes of molecular mass standard proteins (lane Std) are indicated on the left.
Purification of cyanophycin synthetase.
Cyanophycin synthetase was enriched 123-fold to electrophoretic homogeneity from the soluble protein fraction of E. coli(pSK::cphAMA19co) by anion-exchange chromatography, affinity chromatography, and gel filtration chromatography (Table 3) and accounted for 0.8% (wt/wt) of the total protein in the soluble cell fraction. SDS-PAGE of the purified preparation produced one protein band, which corresponded to a molecular mass of 100 ± 15 kDa (Fig. 2a, lane 7). This value was in good agreement with the calculated molecular mass of the translation product of cphA (100.6 kDa).
TABLE 3.
Purification of cyanophycin synthetase from recombinant E. coli harboring cphA of Synechococcus sp. strain MA19
| Purification step | Vol (ml) | Protein (mg) | Total activity (U)a | Sp act (U mg of protein−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Soluble protein fraction | 90.0 | 1,703 | 1,566 ± 1.5 | 0.9 ± 0.5 | 1.0 | 100 |
| Q-Sepharose | 95.0 | 164 | 1,166 ± 2.0 | 7.1 ± 1.0 | 7.7 | 74.5 |
| Procion Blue | 10.0 | 11 | 254 ± 1.5 | 23.1 ± 0.5 | 25.1 | 15.8 |
| Superdex S200 | 10.5 | 1.8 | 203 ± 1.0 | 113.0 ± 1.0 | 123.0 | 13.0 |
One unit was defined as incorporation of 1 nmol of l-arginine min−1.
Characterization of the in vitro polyamide synthesis catalyzed by cyanophycin synthetase.
Purified cyanophycin synthetase requires cyanophycin as a primer and is almost completely inactive without this compound (reference 1 and references therein). Cyanophycin synthetase purified from recombinant cells of E. coli harboring pSK::cphAMA19co used only cyanophycin, modified cyanophycin, and the α-arginyl aspartic acid dipeptide as primers for elongation of the cyanophycin polyamide chain (Table 4). Impure enzyme also catalyzed in vitro cyanophycin synthesis by using noncyanophycin primers, such as N-acetylglucosamine and chemosynthetic poly-α,β-dl-aspartic acid, as well as cyanophycin-free cell membrane fragments of Synechococcus sp. strain PCC7942 and aspartic acid-rich exobiopolymer from Klebsiella sp. (Table 4). While 45% activity was expressed when lot 107FO334 α-arginyl aspartic acid dipeptide was used as the primer, only 2% activity was expressed with lot 125HO515 dipeptide (Table 4). The reason for the difference is not known. The molecular size of the biopolymers obtained when lot 107FO334 dipeptide was used was similar to the molecular size of cyanophycin isolated from the E. coli recombinant strain harboring cphAMA19co (Fig. 2a, lane 6, and Fig. 2b, lane 1).
TABLE 4.
Primer specificity of the purified cyanophycin synthetase expressed from E. coli haboring pSK::cphAMA19coa
| Primer added | Relative activity (%)b
|
|
|---|---|---|
| Soluble protein fraction | Purified enzyme | |
| Cyanophycin | 100.0 ± 0.2 | 100.0 ± 0.5 |
| Modified cyanophycinc | 120.0 ± 1.5 | 140.0 ± 2.5 |
| None | 4.0 ± 2.0 | 0.1 ± 0.1 |
| Poly-α,β-dl-aspartic acidd | 8.7 ± 0.5 | 6.9 ± 1.5 |
| Poly-α-l-aspartic acid | 0.8 ± 0.5 | NDe |
| N-Acetylglucosamine | 90.0 ± 2.5 | 1.8 ± 2.0 |
| α-Arginyl aspartic acid dipeptide (lot 197FO334) | 75.0 ± 1.0 | 45.0 ± 2.5 |
| α-Arginyl aspartic acid dipeptide (lot 125HO515) | 10.0 ± 1.5 | 2.0 ± 0.5 |
| Cell membrane fraction from Synechococcus sp. strain PCC7942 | 14.0 ± 1.0 | ND |
| Aspartic acid-rich exobiopolymer from Klebsiella sp. | 80.0 ± 0.5 | ND |
The reaction conditions were those described in Materials and Methods, except that the primer was varied. The primers were each added at a final concentration of 1 mg ml−1. The experiments were done in triplicate, and means ± standard errors (P < 0.05) were determined.
The relative activities were calculated with respect to the control (when purified cyanophycin from Synechococcus sp. strain MA19 was used as the primer); 100% activity for the unpurified enzyme corresponded to a specific activity of 1.1 U mg of protein−1, and 100% activity for the purified enzyme corresponded to a specific activity of 128 U mg of protein−1. One unit was defined as incorporation of 1 nmol of arginine per min.
The modified cyanophycin contained aspartic acid and arginine at a molar ratio of approximately 1:0.6.
The exact structure of polyaspartic acid LPOC618 obtained by thermal condensation is not known.
ND, not detectable.
The products obtained from the enzyme reactions in which noncyanophycin substances were used as primers were analyzed by SDS-PAGE (Fig. 2a). When chemosynthetic poly-α,β-dl-aspartic acid (Fig. 2a, lane 2), N-acetylglucosamine (Fig. 2a, lane 3), or lot 107FO334 α-arginyl aspartic acid dipeptide (Fig. 2a, lane 4) was used as the primer, the cyanophycin synthetase-containing soluble cell extracts synthesized polymeric material that had a molecular mass of 28 to 30 kDa, which corresponded to the molecular mass of cyanophycin purified from recombinant E. coli (Fig. 2b, lane 1) and clearly differed from the molecular mass of an authentic cyanobacterial sample (Fig. 2a, lane 5). A reduced amount of polymer was also detected after elongation of α-arginyl aspartic acid dipeptide when purified enzyme was used (Fig. 2a, lane 6). In a complete 2-ml reaction mixture containing excess ATP (9 μmol) when cyanophycin was the primer, the purified cyanophycin synthetase (40 μg ml−1) incorporated approximately 5 μmol of arginine and 5 μmol of aspartic acid into the growing cyanophycin chain during incubation for 20 h. There was no l-[U-14C]arginine incorporation if CTP or GTP was used instead of ATP (data not shown).
To investigate the role of phosphorylation steps in the enzyme reaction catalyzed by cyanophycin synthetase, the effect of two acidic phosphatases on incorporation of arginine was examined. We found that [14C]arginine incorporation was stopped rapidly when we added the acidic pyrophosphatase (EC 3.6.1.1) or orthophosphoric phosphohydrolase-phosphatase (acid optimum) (EC 3.1.3.2) (Boehringer) at a concentration of approximately 5 μg ml−1 to the reaction mixtures.
Substrate specificity.
Table 5 shows the activities of the Synechococcus sp. strain MA19 cyanophycin synthetase in response to various substrates. The specific activities of the cyanophycin synthetase were almost identical whether l-[14C]arginine or l-[14C]aspartic acid was used as the labeled substrate (Table 5, reactions 1 and 13). The ratio of the incorporation rate of l-aspartic acid to the incorporation rate of l-arginine (1:0.95) corresponded to the ratio of the two amino acids in authentic cyanophycin. Only negligible activities were found when l-aspartic acid or l-arginine was omitted. No activity was detected when arginine was replaced by l-glutamic acid, α-arginyl aspartic acid dipeptide, citrulline, ornithine, arginine amide, agmatine, or norvaline. In the reaction in which lysine replaced arginine, negligible enzyme activity was detected (Table 5, reaction 9). However, when β-hydroxy aspartic acid was used instead of l-aspartic acid, about 83% enzyme activity was obtained (Table 5, reaction 6).
TABLE 5.
Substrate specificity of purified cyanophycin synthetase from recombinant E. coli (pSK::cphAMA19co)
| Reaction | Variation of substrate mixturea | Labeled substrate usedb | Relative activity (%)c |
|---|---|---|---|
| 1 | Completec | l-[U-14C]arginine | 100 ± 2.5 |
| 2 | Without l-aspartic acid | l-[U-14C]arginine | 2.0 ± 1.0 |
| 3 | Without l-arginine | l-[U-14C]aspartic acid | 5.0 ± 0.5 |
| 4 | l-Glutamic acid instead of l-aspartic acid | l-[U-14C]arginine | 3.0 ± 0.5 |
| 5 | l-Glutamic instead of l-arginine | l-[U-14C]glutamic acid | NDd |
| 6 | β-Hydroxy-l-aspartic acid instead of l-aspartic acid | l-[U-14C]arginine | 83.0 ± 2.5 |
| 7 | α-Arginyl aspartic acid dipeptide lot 107FO334 instead of l-aspartic acid | l-[U-14C]arginine | 4.0 ± 1.5 |
| 8 | α-Arginyl aspartic acid dipeptide lot 107FO334 instead of l-arginine | l-[U-14C]aspartic acid | ND |
| 9 | l-Lysine instead of l-arginine | l-[3H]lysine | 2.7 ± 1.0 |
| 10 | l-Lysine instead of l-aspartic acid | l-[3H]lysine | ND |
| 11a | l-Canavanine instead of l-arginine | l-[U-14C]aspartic acid | 62.0 ± 3.0 |
| 11b | l-Canavanine instead of l-arginine | l-[3H]canavanine | 58.0 ± 2.0 |
| 12 | l-Canavanine added | l-[U-14C]arginine | 100.6 ± 2.5 |
| 13 | Complete | l-[U-14C]aspartic acid | 105.7 ± 2.0 |
| 14 | l-Canavanine added | l-[U-14C]aspartic acid | 158.6 ± 1.5 |
| 15 | l-Citrulline instead of l-arginine | l-[U-14C]aspartic acid | ND |
| 16 | l-Ornithine instead of l-arginine | l-[U-14C]aspartic acid | ND |
| 17 | l-Arginine amide instead l-arginine | l-[U-14C]aspartic acid | ND |
| 18 | l-Agmatine instead of l-arginine | l-[U-14C]aspartic acid | ND |
| 19 | l-Norvaline instead of l-arginine | l-[U-14C]aspartic acid | ND |
The complete reaction mixture is described in Materials and Methods.
The concentrations of the radiolabeled compounds used were 10 μM for l-[U-14C]aspartic acid and 5 μM for the other compounds. The experiments were done in triplicate, and means ± standard errors (P < 0.05) were determined.
A relative activity of 100% corresponded to a specific activity of 125.3 U mg of protein−1. One unit was defined as incorporation of 1 nmol of arginine per min.
ND, not detectable.
Replacement of l-arginine by 0.5 mM l-canavanine, which was shown to be an arginine analogue and a competitive inhibitor of the cyanophycin synthetase from Synechocystis sp. strain PCC6308 (1), resulted in a residual activity of 62%, which corresponded to a rate of incorporation of [3H]canavanine of 58%. As incorporation of canavanine is competitive with incorporation of arginine, it can be totally overcome by equal amounts of arginine (Table 5, reaction 12). On the other hand, the rate of incorporation of l-aspartic acid in a complete reaction mixture in the presence of additional canavanine (Table 5, reaction 14) was 159%, which corresponded to the sum of the activities in reactions 13 and 11a (Table 5).
Cyanophycin synthetase isolated from recombinant E. coli had the parental thermostable character.
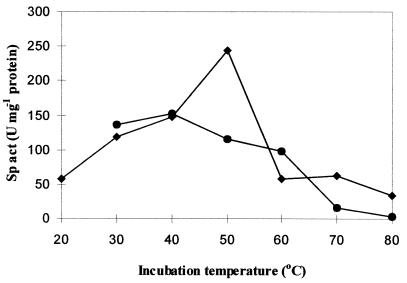
Maximum activity was obtained at an assay temperature of 50°C; this was also observed previously for the enzyme isolated from the parental cyanobacterium Synechococcus sp. strain MA19 (8). The activity decreased at higher assay temperatures. If the enzyme was preincubated for 30 min at an elevated temperature and then activity was assayed at 30°C, 84 and 72% of the activity remained after preincubation at 50 and 60°C, respectively. A level of enzyme activity of 100% at an incubation temperature of 30°C corresponded to a specific activity of 136 U mg of protein−1 (Fig. 3).
FIG. 3.
Temperature profile (⧫) and thermostability (•) of the purified cyanophycin synthetase (40 μg ml−1). To determine the temperatuSre profile, the enzyme activity was assayed at 20, 30, 40, 50, 60, 70, and 80°C for 15 min. The thermostability of the enzyme was determined by incubating the enzyme solution at different temperatures (30, 40, 50, 60, 70, and 80°C) for 30 min and then measuring the activity at 30°C as described in Materials and Methods.
Ectoine, which is a nontoxic amphoterous protector and stabilizer of enzymes and proteins (16), significantly stabilized the cyanophycin synthetase. In the presence of 1 M ectoine the enzyme retained approximately 90% of its activity even after 120 min of incubation at 50°C. In the absence of ectoine only 45% of the enzyme activity was retained. The rate of incorporation of l-[U-14C]arginine at the beginning of incubation represented 100% enzyme activity (128 U mg of protein−1).
DISCUSSION
By combining the methods of colony hybridization with PCR and inverse PCR, the complete cphBA locus encoding cyanophycinase and cyanophycin synthetase was identified and cloned. As in other cyanobacteria, cphA was located downstream of and colinear with cphB in the genome of Synechococcus sp. strain MA19. All regions which had been identified previously in the CphA consensus sequence as possible important sites of cyanophycin synthetase (1, 3) were also located in Synechococcus sp. strain MA19 CphA. The multiple alignment identified only 71 positions in the Synechococcus sp. strain MA19 cphA translation product that were unique to this sequence. In the resulting phylogram constructed for six available cyanophycin synthetases, Synechococcus sp. strain MA19 grouped with S. elongatus and A. variabilis in a distinct cluster.
Polymeric material that accounted for up to 25% (wt/wt) of the cellular dry matter was isolated from cells harboring pSK::cphAMA19co. This polyamide was composed of only aspartic acid and arginine, which is in accordance with the composition of the authentic cyanobacterial polymer, and it differed from cyanophycin-like polymers isolated from other recombinant strains of E. coli, which contained lysine in addition to these two amino acids (39).
The cyanophycin synthetase activities in the recombinant strains of E. coli depended on the orientation of the gene with respect to the lacPO region. The CphA enzyme was overexpressed and active when the orientation of the corresponding cphA gene was colinear with respect to lacPO. No activity was detected when the gene was transcribed from pSK::cphAMA19anti. Hence, the cloned DNA fragment contained a functional active cphA gene but no promoter which can be recognized in E. coli.
The cyanophycin synthetase was purified to electrophoretic homogeneity from E. coli harboring pSK::cphAMA19co. The purified enzyme exhibited more restricted primer selectivity than the impure enzyme in the crude protein soluble fraction. The purified form accepted only cyanophycin, modified cyanophycin, and the α-arginyl aspartic acid dipeptide as primers for elongation of the cyanophycin polyamide chain. However, the unpurified enzyme catalyzed in vitro polymerization by using noncyanophycin primers such as N-acetylglucosamine and poly-α,β-dl-aspartic acid, as well as cyanophycin-free cell membrane fragments of Synechococcus sp. strain PCC7942 and aspartic acid-rich exobiopolymer from Klebsiella sp. Perhaps these macromolecules fulfill a function as nuclear crystals for growth of the cyanophycin polyamide chain by acting as binding agents for the cyanophycin oligomers, including dimers and trimers, which presumably were present in the soluble protein fraction of the recombinant cells, thus permitting the elongation reaction. This would be consistent with the reaction mechanism postulated recently (3). No de novo cyanophycin synthesis occurred in the reactions catalyzed by purified cyanophycin synthetase in the absence of cyanophycin primers or in the presence of noncyanophycin primers. The use of α-arginyl aspartic acid dipeptide, in which both carboxylic groups of the aspartic acid residue are free, as a primer for polyamide de novo synthesis produced different results, which depended on the batch of dipeptide purchased. Since no detailed analysis of the two batches was available from the manufacturer, the reason for the difference and the catalytic mechanism for this primer type remain unknown.
Compared to other cyanophycin synthetases described recently (3), the enzyme from a recombinant strain of E. coli harboring cphAMA19co exhibited differences not only in primer selectivity but also in substrate specificity. Only one of the intermediates of the urea cycle and arginine analogues, canavanine, served as an alternative substrate for cyanophycin synthetase and was incorporated instead of l-arginine. Whether incorporation of canavanine occurred in one growing polymer chain or occurred independently at separate primer sites (i.e., whether a copolymer or a blend of homopolymers was formed) remains to be elucidated. The in vivo influence of canavanine on cyanophycin accumulation was demonstrated in a previous study (21). After addition of the arginine analogue to the medium, formation of akinets increased fivefold and there was a concomitant complete loss of cyanophycin granules. The data obtained by us showed that canavanine affected in vitro cyanophycin synthesis by competitive incorporation.
The purified cyanophycin synthetase had a temperature profile and a thermostability like those of the enzyme preparation which was obtained from the parental cyanobacterium, Synechococcus sp. strain MA19 (8). The stability could be improved by addition of ectoine, thus making the enzyme from Synechococcus sp. strain MA19 a promising candidate for in vitro cyanophycin and polyamide production on a technical scale.
Acknowledgments
We thank M. Miyake and Y. Asada (Molecular Laboratory of the National Institute of Bioscience and Human Technology, Tsukuba, Ibaraki, Japan) for providing Synechococcus sp. strain MA19 and E. Galinski (Institut für Biochemie, Westfälische Wilhelms-Universität, Münster, Germany) for providing ectoine. The assistance of D. Rehder with inverse PCR is gratefully acknowledged.
REFERENCES
- 1.Aboulmagd, E., F. B. Oppermann-Sanio, and A. Steinbüchel. 2000. Molecular characterization of the cyanophycin synthetase from Synechocystis sp. strain PCC6308. Arch. Microbiol. 174:297–306. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. M. 1984. Cyanobacterial cell inclusions. Annu. Rev. Microbiol. 38:1–25. [DOI] [PubMed] [Google Scholar]
- 3.Berg, H., K. Ziegler, K. Piotukh, K. Baier, W. Lockau, and R. Volkmer-Engert. 2000. Biosynthesis of the cyanobacterial reserve polymer multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Mechanism of the cyanophycin synthetase reaction studied with synthetic primers. Eur. J. Biochem. 267:5561–5570. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borzi, A. 1887. Le comunicazioni intracellulari delle Nostochinee. Malpighia 1:28–203. [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. [DOI] [PubMed] [Google Scholar]
- 7.Eveland, S. S., D. L. Pompliano, and M. S. Anderson. 1997. Conditionally lethal Escherichia coli murein mutants contain point defects that map to regions conserved among murein and folyl poly-γ-glutamate ligases: identification of a ligase superfamily. Biochemistry 36:6223–6229. [DOI] [PubMed] [Google Scholar]
- 8.Hai, T., F. B. Oppermann-Sanio, and A. Steinbüchel. 1999. Purification and characterization of cyanophycin and cyanophycin synthetase from the thermophilic Synechococcus sp. MA19. FEMS Microbiol. Lett. 181:229–236. [DOI] [PubMed] [Google Scholar]
- 9.Hein, S., T. Hai, and A. Steinbüchel. 1998. Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch. Microbiol. 170:162–170. [DOI] [PubMed] [Google Scholar]
- 10.Herdman, M. 1987. Akinetes: structure and function, p.227–250. In P. Fay and C. van Baalen (ed.), The cyanobacteria. Elsevier, Amsterdam, The Netherlands.
- 11.Joentgen, W., T. Groth, A. Steinbüchel, T. Hai, and F. B. Oppermann. January 2001. Polyaspartic acid homopolymers and copolymers, biotechnological production and use thereof. U.S. patent 6,180,752 B1.
- 12.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:185–209. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685. [DOI] [PubMed] [Google Scholar]
- 14.Lawry, N. H., and R. D. Simon. 1982. The normal and induced occurrence of cyanophycin inclusion bodies in several blue-green algae. J. Phycol. 18:391–399. [Google Scholar]
- 15.Liotenberg, S., D. Campbell, R. Rippka, J. Houmard, and N. T. de Marsac. 1996. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology 142:611–622. [DOI] [PubMed] [Google Scholar]
- 16.Lippert, K., and S. Galinski. 1992. Enzyme stabilization by ectoine-type compatible solutes, protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61–65. [Google Scholar]
- 17.Mackerras, A. H., N. M. De Chazal, and G. D. Smith. 1990. Transient accumulations of cyanophycin in Anabaena cylindrica and Synechocystis PCC6308. J. Gen. Microbiol. 136:2057–2065. [Google Scholar]
- 18.Makino, S. J., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrit, M. V., S. S. Sid, L. Mesh, and M. M. Allen. 1994. Variation in the amino acid composition of cyanophycin in the cyanobacterium Synechocystis sp. PCC6308 as a function of growth condition. Arch. Microbiol. 162:158–166. [DOI] [PubMed] [Google Scholar]
- 20.Miyake, M., M. Erata, and Y. Asada. 1996. A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J. Ferment. Bioeng. 82:512–514. [Google Scholar]
- 21.Nichols, J. M., D. G. Adams, and N. G. Carr. 1980. Effect of canavanine and other amino acid analogues on akinete formation in the cyanobacterium Anabaena cylindrica. Arch. Microbiol. 127:67–75. [Google Scholar]
- 22.Oppermann-Sanio, F. B., T. Hai, E. Aboulmagd, F. F. Herzayen, S. Jossek, and A. Steinbüchel. 1999. Biochemistry of microbial polyamide metabolism, p.185–193. In A. Steinbüchel (ed.), Biochemical principles and mechanisms of biosynthesis and biodegradation of polymers. Wiley-VCH, Weinheim, Germany.
- 23.Richter, R., M. Hejazi, R. Kraft, K. Ziegler, and W. Lockau. 1999. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartic acid (cyanophycin). Molecular cloning of the gene of Synechocystis sp. PCC6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur. J. Biochem. 263:163–169. [DOI] [PubMed] [Google Scholar]
- 24.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61. [Google Scholar]
- 25.Page, R. D. M. 1996. TreeView: an application to display phylogenetic tree in personal computer. Comput. Applic. Biosci. 12:357–358. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Schwamborn, M. 1998. Chemical synthesis of polyaspartates: a biodegradable alternative to currently used polycarboxylate homo- and copolymers. Polym. Degrad. Stabil. 59:39–45. [Google Scholar]
- 28.Simon, R. D. 1971. Cyanophycin granules from the blue-green algae Anabaena cylindrica: a reserve material consisting of copolymers of aspartic acid and arginine. Proc. Natl. Acad. Sci. USA 68:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon, R. D. 1973. The effect of chloramphenicol on the production of cyanophycin granule polypeptide in the blue-green algae Anabaena cylindrica. Arch. Microbiol. 92:115–122. [DOI] [PubMed] [Google Scholar]
- 30.Simon, R. D., and P. Weathers. 1976. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim. Biophys. Acta 420:165–176. [DOI] [PubMed] [Google Scholar]
- 31.Simon, R. D. 1976. The biosynthesis of multi-l-arginyl-poly(l-aspartic acid) in the filamentous cyanobacterium Anabaena cylindrica. Biochim. Biophys. Acta 422:407–418. [DOI] [PubMed] [Google Scholar]
- 32.Simon, R. D. 1987. Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedral bodies, p.199–225. In P. Fay and C. Van Baalen (ed.), The cyanobacteria. Elsevier, Amsterdam, The Netherlands.
- 33.Stanier, R. Y., and G. Cohen-Basire. 1977. Phototrophic prokaryotes: the cyanobacteria. Annu. Rev. Microbiol. 31::225–274. [DOI] [PubMed] [Google Scholar]
- 34.Suarez, C., S. J. Kohler, M. M. Allen, and N. H. Kolodny. 1999. NMR study of the metabolic 15N isotopic enrichment of cyanophycin synthesized by the cyanobacterium Synechocystis sp. strain PCC6308. Biochim. Biophys. Acta 1426:429–438. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland, J. M., J. Reaston, W. D. P. Stewart, and M. Herdman. 1985. Akinetes of the cyanobacterium Nostoc PCC 7524. Macromolecular and biochemical changes during synchronous germination. J. Gen. Microbiol. 131:2855–1863. [Google Scholar]
- 36.Thoden, J. B., T. J. Kappock, J. A. Stubbe, and H. M. Holden. 1999. Three-dimensional structure of N5-carboxyaminoimidazole ribonucleotide synthetase: a member of the ATP grasp protein superfamily. Biochemistry 38:15480–15492. [DOI] [PubMed] [Google Scholar]
- 37.Triglia, T., M. G. Peterson, and D. J. Kemp. 1988. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 16:8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Eykelenburg, C. 1979. The ultrastructure of Spirulina platensis in relation to temperature and light intensity. Antonie Leeuwenhoek 45:369–390. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler, K., A. Diener, C. Herpin, R. Richter, R. Deutzmann, and W. Lockau. 1998. Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartate (cyanophycin). Eur. J. Biochem. 254:154–159. [DOI] [PubMed] [Google Scholar]