Abstract
During mouse eye development, the correct formation of the lens occurs as a result of reciprocal interactions between the neuroectoderm that forms the retina and surface ectoderm that forms the lens. Although many transcription factors required for early lens development have been identified, the mechanism and genetic interactions mediated by them remain poorly understood. Foxe3 encodes a winged helix-forkhead transcription factor that is initially expressed in the developing brain and in the lens placode and later restricted exclusively to the anterior lens epithelium. Here, we show that targeted disruption of Foxe3 results in abnormal development of the eye. Cells of the anterior lens epithelium show a decreased rate of proliferation, resulting in a smaller than normal lens. The anterior lens epithelium does not properly separate from the cornea and frequently forms an unusual, multilayered tissue. Because of the abnormal differentiation, lens fiber cells do not form properly, and the morphogenesis of the lens is greatly affected. The abnormally differentiated lens cells remain irregular in shape, and the lens becomes vacuolated. The defects in lens development correlate with changes in the expression of growth and differentiation factor genes, including DNase II-like acid DNase, Prox1, p57, and PDGFα receptor. As a result of abnormal lens development, the cornea and the retina are also affected. While Foxe3 is also expressed in a distinct region of the embryonic brain, we have not observed abnormal development of the brain in Foxe3−/− animals.
Development of the vertebrate eye requires a coordinated development of the retina and the lens. Interaction between the lens and retina has been investigated since the beginning of the last century (45) and has led to the following understanding of eye formation. Anterior neuroectoderm, from which the retina is derived, is responsible for the induction of the lens, and the lens is responsible for the placement of the retina and induction of the cornea (4, 10, 52). Typically, an abnormal development of the retina or the lens leads to abnormal morphogenesis of the entire eye, resulting in diminished visual function. For this reason, identification of key components necessary for the development of either structure is of critical importance.
Several genes have been identified that play important roles in the development of the lens. A key component of the lens-forming cascade is the homeodomain-containing transcription factor Pax6. Pax6 is expressed in the retina as well as in the lens, but it is its expression in the lens that is essential for lens formation. Mice that lack Pax6 expression in the head ectoderm do not develop a lens (2, 20). Several downstream targets of Pax6 have been identified, and mutations in these genes frequently lead to abnormal development of the anterior eye segment (10, 12). In mouse, one of the downstream targets of Pax6 is the Fox gene Foxe3 (5, 8, 14). Fox genes, previously known as forkhead or winged helix genes (22), encode transcription factors that can act as activators or repressors in regulation of embryonic pattern formation, tissue-specific gene expression, and tumorogenesis (1, 9, 15, 16, 18, 19, 23, 26-29, 46). These proteins contain a highly conserved 110-amino-acid DNA binding domain (27, 47). Several of them are involved in eye formation. Foxg1 is a forkhead gene expressed in the nasal half of the retina, and its elimination results in abnormal eye development (50). In contrast, Foxd1 is expressed in the temporal retina (21) and together with Foxg1 controls the formation of the retinotectal map (50a). Mutations in FOXC1 and FOXC2 result in aberrant development of the anterior chamber of the eye and glaucoma in humans (25, 34, 44). Foxn4 is expressed in a subset of retinal progenitor cells, and it controls the genesis of amacrine and horizontal cells (30). Foxl2 is involved in eyelid formation (11, 13).
Foxe3 expression begins in the lens placode at embryonic day 9.5 (E9.5). During the differentiation of the lens, Foxe3 expression remains in the anterior lens epithelium (ALE) but is turned off in the differentiating lens fiber cells. By E14.5 the Foxe3 expression is limited to the ALE, where it persists into adulthood. In addition to the developing lens, Foxe3 is also expressed in two distinct areas in the forebrain-midbrain boundary (5, 8). This expression begins at E8.5 and ceases around E12.5. The function of the Foxe3 protein seems to be important for lens development as mutations affecting the structure of this protein cause developmental defects in lens formation. Foxe3 is mutated in the spontaneous mouse mutation dysgenetic lens (dyl) (39, 40). dyl/dyl animals have smaller lenses that show several developmental defects (5, 8, 35, 39, 40). In humans, a mutation in the C-terminal region of the FOXE3 gene that results in an addition of 111 amino acids to the Foxe3 protein leads to cataracts and accompanying anterior segment ocular dysgenesis typical of Peters' anomaly (37, 42). Additionally, one individual with Peters' anomaly was found to be heterozygous for a G→T mutation, producing an Arg90Leu substitution in the DNA-binding domain of FOXE3 (35, 37). In all of the described cases, a mutant protein is produced in affected individuals.
Since it is not known to what degree the function of Foxe3 is eliminated in these naturally occurring mutants, we decided to study lens development in the absence of Foxe3 function. For this purpose, we created a targeted deletion of the Foxe3 gene. We found that a complete lack of Foxe3 function leads to severe abnormalities in eye formation that are initiated during the development of the lens placode. The cells of the anterior lens epithelium show diminished proliferation, resulting in a smaller lens. At later stages, the cells of the anterior lens epithelium initiate the differentiation inappropriately early. However, these cells do not differentiate properly. Fiber cells do not acquire the typical spindle-formed morphology, they do not loose their nuclei, and the lens eventually develops several vacuoles and a cataract. Because of the aberrant development of the lens, the retina shrinks and folds abnormally. As a result the affected animals have smaller eyes, which in some cases remain closed during their entire life.
MATERIALS AND METHODS
A targeted deletion of Foxe3.
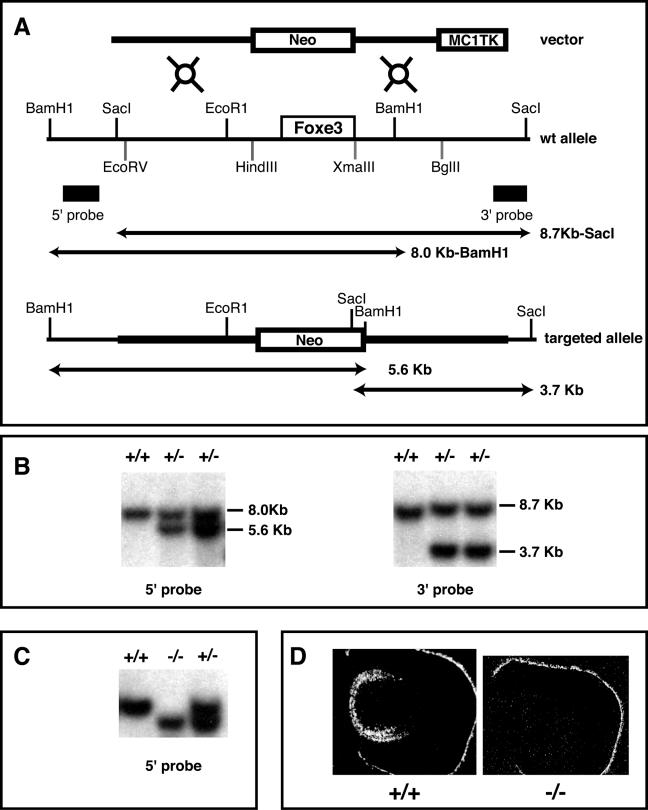
We generated Foxe3 null mice using the strategy depicted in Fig. 1A. Briefly, a 3.1-kb EcoRV-HindIII 129/Sv genomic fragment containing the 5′ flanking DNA of the Foxe3 gene was subcloned with the PGK-lox-Neo-containing cassette in the same transcriptional orientation as the Neo gene. A 2.4-kb XmaIII-BglII genomic fragment that contains the 3′ end of the Foxe3 open reading frame plus flanking sequences was cloned into the pMC1TK (where TK is thymidine kinase) cassette and excised by SacII. This 2.4-kb MC1TK fragment was inserted into the SacII site at the 3′ end of the 3.1-kb PGK-lox-Neo cassette. This targeting vector contains Foxe3 upstream and downstream sequences, but the open reading frame is replaced by a PGK-lox-Neo cassette. This vector was linearized with ClaI and electroporated into AB1 embryonic stem cells (31). During homologous recombination this construct replaced the Foxe3 coding region. Embryonic stem cells positive for replacement of the Foxe3 gene by the targeting vector were injected into C57B6 blastocysts. Chimeric mice were born as identified by their coat color. Genotypes of mouse embryos were determined by Southern blotting using genomic DNA digested by BamHI (5′ probe) or SacI (3′ probe). Mice in this study were analyzed on a B6 × 129 mixed genetic background.
FIG. 1.
Generation of the Foxe3−/− mice. (A) Diagram representing the targeting vector used for mutagenesis (top), the Foxe3 locus (middle), and the Foxe3 targeted allele (bottom). Open boxes represent the Foxe3 coding region, the Neo and TK cassettes. Rectangles represent the 5′ and 3′ probes used for Southern screening. Predicted fragments for BamHI and SacI digestion before and after homologous recombination are illustrated as lines with arrowheads. (B) Southern blot of two targeted clones using BamHI and the 5′ probe and SacI and the 3′ probe. (C) Southern blot genotyping of tail DNA from progeny of heterozygous mice after BamHI digestion. (D) In situ hybridization of Foxe3 at E14.5 showing the expression of this gene in sections of wild type embryos (+/+) and its absence in Foxe3−/− embryos (−/−).
Histology.
Embryos were fixed in 4% paraformaldehyde, dehydrated in ethanol, and cleared in xylene. After three 2-h incubations in paraplast, they were mounted and sectioned. The sections were dewaxed, rehydrated, and stained with hematoxylin and eosin.
BrdU incorporation.
To analyze levels of proliferation in the developing embryos, pregnant females were injected intraperitoneally with 100 μg/gram of body weight of 5-bromo-2′-deoxyuridine (BrdU). After 2 h, animals were sacrificed, and embryos were fixed overnight in 10% formalin. The next day the embryos were dehydrated in a graded series of ethanol. Paraffin-embedded sections were microwaved in 10 mM sodium citrate, pH 6.0, for antigen retrieval. BrdU was detected with an anti-BrdU-fluorescein isothiocyanate antibody (Becton Dickinson).
In situ hybridization.
Whole-mount and section in situ hybridizations were performed using standard protocols (49). Probes were labeled by using digoxigenin or S35, respectively, by in vitro transcription using either T7 or T3 RNA polymerase.
Semiquantitative PCR.
Total RNA was isolated from wild-type or mutant lens obtained from E17.5 embryos using an RNeasy kit (QIAGEN). Two micrograms of total RNA was reverse transcribed with Superscript II reverse transcriptase and random hexameres. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin was performed as a control to demonstrate that there was a comparable amount of mRNA in both groups. The primers used in the semiquantitative PCR were as follows: DNase II-like acid DNase (DLAD), 5′CCAGTTCATGGCTATGAGTAC3′ and 5′TTAGGTCTCCAATGCAGGTCCAGCGATTTG3′; β-actin, 5′TGTGATGGTGGGAATGGGTCAG3′ and 5′TTTGATGTCACGCACGATTTCC3′; GAPDH, 5′CAATGTGTCCGTCGTGGATCT3′ and 5′GCCTGCTTCACCACCTTCCTT3′ (33). To demonstrate that there were the same levels of lens-specific mRNA in both groups, amplification of αA-crystallin was detected using the following primers: 5′ACAACGAGAGGCAGGATGAC3′ and 5′AGGGGACAACCAAGGTGAG3′ (3).
RESULTS
We made Foxe3−/− mice using the strategy depicted in Fig. 1A and described in detail in Material and Methods. Using Southern blotting, we confirmed that we obtained animals that were genotypically Foxe3−/− (Fig. 1B and C). We used in situ hybridization with an S35-labeled Foxe3 probe to demonstrate that the Foxe3−/− animals do not express any Foxe3 transcripts in the developing lens, confirming that the mutation produces a null allele (Fig. 1D). The heterozygous mice were apparently normal and were crossed to obtain homozygous mutants. Foxe3−/− mice were viable and fertile. Superficial examination indicated eye abnormalities in all Foxe3−/− mice; in some cases the eyes never opened (Fig. 2A and B). Isolated eyes show that Foxe3−/− animals have much smaller eyes than the wild-type mice, the anterior chamber is not formed, and the pupil is markedly smaller (Fig. 2C and D).
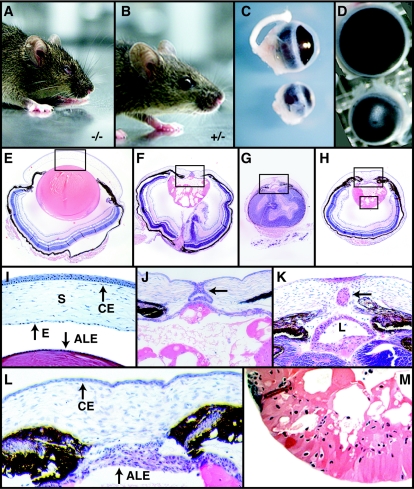
FIG. 2.
External features and histological analysis of Foxe3 mutant mice. Appearance of adult Foxe3−/− (A) and Foxe3+/−mice (B). Lateral (C) and frontal (D) view of adult eyes showing the smaller size of Foxe3−/− eye. (E) Hematoxylin-eosin staining of coronal section of P14 eyes from a wild-type mouse. (F to H) Coronal sections of eyes from P14 Foxe3−/− mice showing abnormal features of the lens, cornea, and retina. The lens in the wild-type animal (E) is bigger than the lens of Foxe3−/− mice (F, G, and H), and the retina displays unusual folding. (I) A high magnification of boxed-in area in panel E shows a well-formed anterior chamber, an endothelial corneal layer along the posterior margin of the cornea, and a single layer of cells in the anterior lens. Pups lacking Foxe3 function display keratolenticular connection (J, arrow), absence of the anterior segment (J to L), and an ectopic lens (K, arrow). The anterior lens epithelium is frequently multilayered (K and L). The lens is heavily vacuolated (M) and nuclei are present in the lens fiber cells (M). Endothelial layer of the cornea is absent and the corneal epithelium is thinner (L). Abbreviations: CE, corneal epithelium; L, lens; E, corneal endothelium; S, stroma.
When the morphology of eyes in Foxe3−/− embryos (Fig. 2F to H) is compared to a section of a wild-type eye (Fig. 2E), several striking changes are apparent. First of all, the lenses in Foxe3−/− embryos are significantly smaller than in the wild type. In addition these lenses contain many vacuoles that disrupt the morphology of the lenses. The retina in these embryos displays abnormal folding that leads to shrinkage of the eye (Fig. 2G). Detailed analysis of eye sections from Foxe3−/− embryos shows several abnormalities. While in the wild-type embryos the single-layered anterior lens epithelium is clearly separated from the cornea (Fig. 2I), in Foxe3−/− embryos, the anterior lens epithelium does not properly separate from the cornea, resulting in the absence of the anterior chamber (Fig. 2J to L). In some cases there is a visible connection between the lens and cornea (Fig. 2J); in others, a fragment of lens tissue can be found between the lens and cornea (Fig. 2K). In addition, the typically single-layered anterior lens epithelium frequently becomes multilayered in Foxe3−/− eyes postnatally (Fig. 2J to L). Normal fiber cells do not form in Foxe3−/− embryos; rather, in the posterior region of the lens, irregularly shaped nucleated cells are present (Fig. 2K and M) that cannot be observed in the wild-type animals. Severe vacuolization of this area is present in all cases. The abnormal development of the lens has deleterious effects on the development of the cornea and retina. Retinal layers appear to be correctly specified, but abnormal folding of the retina is a common phenomenon. Corneal endothelial layer is absent, while the corneal stroma is disorganized and the corneal epithelium is thinner (compare Fig. 2I and L).
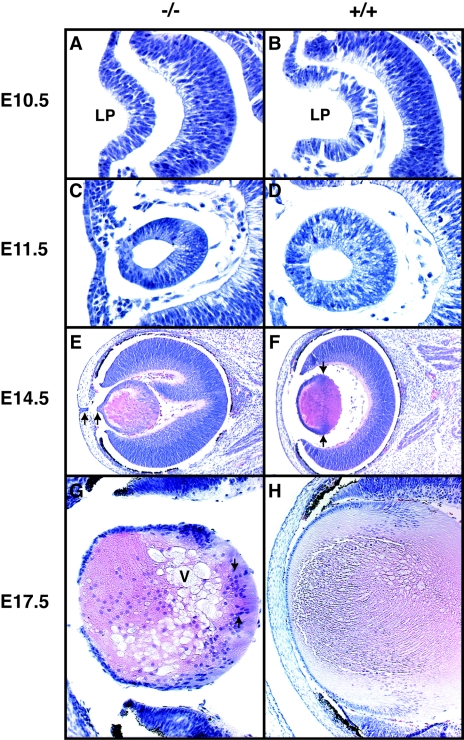
Differences in lens formation between the wild-type and Foxe3−/− embryos can be already observed at E10.5. At this stage the invaginating lens placode is smaller in Foxe3−/− embryos (Fig. 3A) than in the wild-type embryos (Fig. 3B), resulting in a smaller lens at the lens vesicle stage (Fig. 3C and D) and all subsequent stages of development (Fig. 3E to H). At E14.5, the lens in Foxe3−/− embryos begins to be vacuolated (Fig. 3E). The characteristic bow region formed by the lens nuclei that is present in the wild-type lens (Fig. 3F) is either less pronounced or completely absent (Fig. 3E). While there are almost no nuclei in the posterior region of the wild-type lens (Fig. 3F), there are many of them present in the similar region of the Foxe3−/− lens (Fig. 3E and G). A keratolenticular connection, though broken during the preparation of this section, is observable in Fig. 3E. At E17.5, the severely disorganized structure of the lens becomes even more evident (Fig. 3G) in comparison to the wild-type lens (Fig. 3H).
FIG. 3.
Histological analysis of lens development in Foxe3−/− mice. Hematoxylin-eosin stained coronal sections of E10.5 (A and B), E11.5 (C and D), E14.5 (E and F), and E17.5 (G and H) embryonic eyes. At E10.5 the invaginating lens placode (LP) of Foxe3−/− (A) embryos is slightly smaller than the wild-type placode (B). At E11.5 the lens vesicle is smaller in Foxe3−/− embryos (C) than in the wild-type embryos (D). By day E14.5 vacuolar structures start to appear in the Foxe3 mutant (E). The well-defined equatorial layer of nuclei present in the wild-type lens (F, arrows) is not present in the mutant lens (E). In addition, a connection between corneal epithelium and lens epithelium is present in the mutant lens (E, arrows). In this case the narrow connection was broken during the preparation of the slide. At E17.5 the lens of the Foxe3 mutant contains many nuclei in the posterior compartment (G, arrows), while in the wild-type lens nuclei are present only in the anterior half of the lens (H). Note the several large vacuoli (V) in the mutant lens.
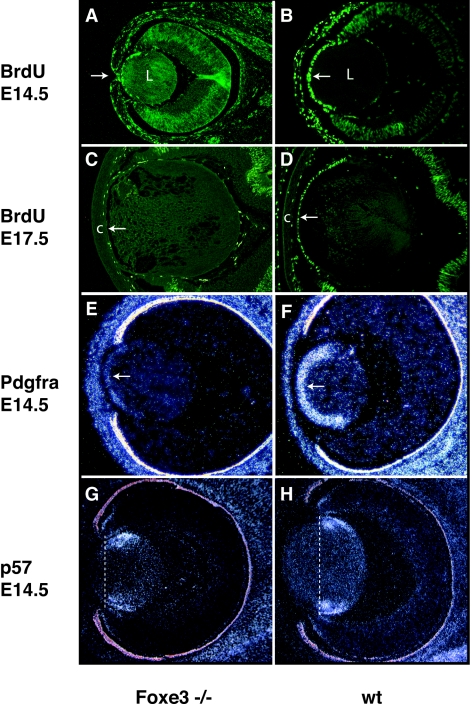
At the molecular level, significant differences can be observed between the proliferation and differentiation of the cells of the anterior lens epithelium in wild-type and Foxe3−/− embryos. Using BrdU incorporation, which is a measure of cell proliferation, we found a strong reduction in cell proliferation in the ALE of Foxe3−/− embryos already at E14.5 (Fig. 4A and B). This difference becomes more pronounced at later stages. At E17.5 there is almost no proliferation in the ALE of Foxe3−/− embryos (Fig. 4C) in comparison to the wild-type ALE (Fig. 3D). This finding is in good agreement with the reduced expression of the platelet-derived growth factor receptor A (Pdgfra) in the ALE of E14.5 Foxe3−/− embryos (Fig. 4E). Pdgfra is typically expressed at high levels in the cells of the ALE (32, 36), and it is believed that PDGF signaling mediated through this receptor is a critical factor driving proliferation of the ALE. In contrast, expression of p57, a protein that mediates cell cycle arrest (51), is shifted anteriorly, leading to premature arrest of proliferation in the ALE (Fig. 4G and H).
FIG. 4.
(A to D) Proliferation in Foxe3−/− and wt lens cells visualized by BrdU incorporation. While at E14.5 in wild-type embryos cells of the anterior lens epithelium (arrow) show high levels of BrdU incorporation (B), there is a reduction of proliferation in Foxe3−/− embryos (A). The arrow in panel A indicates the keratolenticular adhesion. At E17.5 there is almost no BrdU incorporation in the anterior epithelial cells of mutant embryos (C) in comparison to levels in the wild-type embryo (D). While there is a distinct anterior chamber between the anterior lens epithelium (arrow) and the cornea (c) in the wild-type embryo, no such a chamber is present in mutant embryos. (E to H) In situ hybridization of Pdgfra and p57kip to sections of Foxe3−/− and wild-type eyes. In situ hybridization shows that the expression of Pdgfra, a marker of cell proliferation, is significantly reduced in the cells of the anterior lens epithelium (arrow) of Foxe3−/− embryos (E), when compared to the expression in the wild-type anterior lens epithelium (F). At the same time the expression of P57kip2 that is a marker of transition from proliferation to differentiation is also altered. In wild-type lens, p57kip2 is expressed in the equatorial zone (H), while in the Foxe3−/− embryos (G) the expression of p57kip2 is present in a more anterior position. The vertical lines through the lens (G and H) indicate the onset of p57kip2 expression. In all sections, the anterior is to the left. L, lens; c, cornea; wt, wild type.
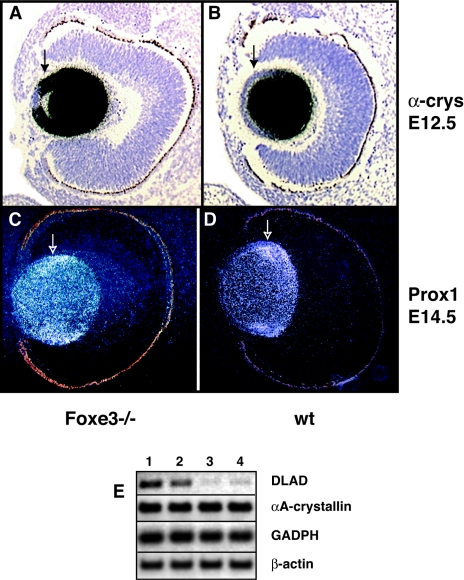
In addition to proliferation, the differentiation of lens cells is also affected in Foxe3−/− embryos. This can be most dramatically demonstrated by the expression of α-crystallin. At E12.5, α-crystallin is expressed at very high levels in the equatorial zone in wild-type lens and differentiated lens but at very low levels in epithelial cells (Fig. 5B). In contrast, in Foxe3−/− embryos, only the most anterior epithelial cells are devoid of α-crystallin expression, while the peripheral regions of ALE express α-crystallin at the same levels as the differentiated lens fiber cells (Fig. 5A). This indicates that the differentiation starts in the cells of the ALE inappropriately early. The incorrect onset of differentiation in Foxe3−/− lenses can also be demonstrated by the expression of the transcription factor Prox1 that is typically expressed in the transition zone from proliferation to differentiation (48). In the wild-type lens, Prox1 expression is mostly present in the equatorial zone (Fig. 5D), while in the Foxe3−/− lens, Prox1 expression is more diffuse and, importantly, present more anteriorly in the ALE (Fig. 5C). This anterior shift of Prox1 expression in the Foxe3−/− embryos is consistent with the previously described expression of the cell-cycle inhibitor p57 that appears to be the downstream target of Prox1 (17). It is somewhat difficult to determine what is the primary cause of the abnormal phenotype in the Foxe3−/− lenses as several cellular processes are affected in this mutant. Figure 5E demonstrates that Foxe3-deficient mice have a lower expression of DLAD than the wild-type siblings. This enzyme is normally expressed at high levels in the lens, and animals lacking this enzyme are unable to degrade their DNA during lens differentiation. They do not loose their nuclei, and they develop cataracts (33). Therefore, the low levels of expression of DLAD might explain the presence of nuclei in the differentiated lens fiber cells of Foxe3−/− embryos.
FIG. 5.
The lens cells in Foxe3 mutants show abnormal patterns of gene expression during differentiation. The expression of α-crystallin in wild-type lens (A) is at very low levels in the epithelial cells but at very high levels in the equatorial zone and differentiated lens. In Foxe3−/− embryos (B), the posterior epithelial cells express α-crystallin at the same level as the differentiated cells, and only the most anterior epithelial cells show a typical, low expression of this gene. Compare similar regions in two embryos indicated by arrows. Prox1, a gene that is typically expressed in the transition zone from proliferation to differentiation, is expressed mainly in the equatorial cells of wild-type lens (C, arrow), while in Foxe3−/− embryos (D), the expression of Prox1 is more diffuse, and high levels of expression extend into the cells of the anterior lens epithelium. (E) Semiquantitative PCR demonstrating strongly reduced levels of DLAD transcripts in lenses of Foxe3−/− (lanes 3 and 4) embryos in comparison to wild-type embryos (lanes 1 and 2). Levels of αA-crystallin, GADPH, and β-actin, which were used as controls, are not changed. wt, wild type.
While Foxe3 is also expressed in a discrete region of the midbrain at E9 to E10.5, we have not observed any abnormalities in brain development of Foxe3−/− embryos (data not shown).
DISCUSSION
During lens development, most of the cells differentiate into lens fiber cells. Only a small population of cells in the anterior of the lens remains relatively undifferentiated and retains its proliferative ability. These are the cells of the anterior lens epithelium. The ratio of the lens fiber cells to the cells of the anterior lens epithelium is carefully controlled by regulating the rate of proliferation and differentiation. One of the genes involved in regulation of these processes is the forkhead domain-containing gene Foxe3. Analysis of lens development in Foxe3−/− mice shows that this gene has a critical function in at least three different events. First of all, it plays an important role in the proliferation of the anterior lens epithelium. By E14.5, the proliferation of the anterior lens epithelium is significantly slowed down in Foxe3−/− embryos and is almost nonexistent by E17.5. Because of this reduced proliferation, the anterior lens epithelium does not generate enough cells to generate a normal-sized lens.
The second important difference between wild-type and Foxe3−/− lenses is that the differentiation of lens fiber cells sets in prematurely in the mutant animals. Finally, the cellular differentiation in Foxe3−/− embryonic lenses does not follow the stereotypic steps present in the wild-type lens. As a result of these three abnormal processes, the cells of the anterior lens epithelium do not form a single layer but, rather, form multilayered structures that do not properly separate from the cornea. The lens fiber cells express typical differentiation products such as crystallins, but they do not develop the characteristic spindle shape, and they do not loose their nuclei. Eventually, the lenses become vacuolated. Since the absence of Foxe3 leads to developmental defects in lens formation that cannot be attributed to only one specific process, it appears that Foxe3 function is an integral part of a regulatory network, which regulates the balance between proliferation and differentiation of lens cells. In the absence of Foxe3 function, cell proliferation is strongly reduced, and differentiation begins prematurely. Several genes are affected, and it is difficult to determine which molecular pathway leads to which specific morphological change. However, it appears that the reduced expression of DLAD is the most likely reason for the lack of elimination of nuclei in the lens fiber cells.
The above-mentioned observations agree well with our previous finding concerning the function of FoxE4 (Xlens1) in Xenopus, which is the functional homologue of Foxe3 (24). During the Xenopus lens development FoxE4 has a pattern of expression very similar to that of Foxe3. It is also expressed in the lens placode and the cells of the anterior epithelium. Overexpression of FoxE4 leads to a reduced differentiation of lens cells, altering the balance in favor of undifferentiated, proliferatively active cells of the anterior lens epithelium (24). The ability of Foxe3/FoxE4 to affect the balance between proliferation and differentiation of cells is not unique among the forkhead genes. For example, in the nude mouse the forkhead transcription factor Foxn1 promotes proliferation of hair follicle epithelial cells and inhibits their differentiation (7, 9, 38). In Xenopus, the forkhead gene FoxG1 (XBF-1) regulates neurogenesis by controlling the proliferation and differentiation of neuronal cells (6). High levels of FoxG1 suppress neuronal differentiation, and low levels of this protein induce neuronal differentiation. Finally, the FoxO subfamily of forkhead genes plays an important role in the balance between proliferation and differentiation (1).
Although many morphological and molecular changes observed in lenses of Foxe3−/− embryos are similar to those observed in the dyl mouse strain (5, 8, 39, 40), there are some clear differences. The most striking is the presence of the multilayered anterior lens epithelium in Foxe3 −/− embryos. This is different from dyl embryos, in which the anterior lens epithelium is diminished (5, 8). While in dyl embryos many cells undergo complete differentiation that includes expression of crystallins, lens fiber elongation, and loss of nuclei, in Foxe3−/− the loss of nuclei and lens fiber elongation is severely disturbed. The most likely explanation for these differences in phenotype is that the dyl allele is not a null mutation but a hypomorphic allele. In this strain, there are two missense mutations in the DNA binding domain of the Foxe3 protein. As a consequence of the missense mutations in the DNA domain, the ability of the Foxe3 dyl protein to bind DNA is largely eliminated (35). However, it is difficult to evaluate to what degree the function of the Foxe3 protein is eliminated in dyl, as a mutant Foxe3 protein is still produced and the direct targets of Foxe3 are unknown.
In humans, a mutation in the evolutionarily conserved C terminus of the FOXE3 gene correlates with anterior segment dysgenesis and cataracts characteristic of Peters' anomaly (37, 42, 43). This mutation creates a frameshift and results in an addition of 111 amino acids to the FOXE3 protein. This mutation in FOXE3 is dominant, indicating that the mutant protein that was created is either involved in dominant-negative interactions or that there is an important regulatory domain in the C-terminal end of the protein and the observed phenotype is a loss-of-function mutation with haploinsufficiency. In addition, one individual with a missense mutation in the DNA-binding domain of FOXE3 has a lens phenotype characteristic of Peters' anomaly (35, 37). Since this phenotype is similar to the phenotype observed in dyl heterozygous mice, it was suggested that the human condition is a haploinsufficiency (35). However, haploinsufficiency is very difficult to prove using human phenotypes (41), and for that reason in all of the above cases, it is not certain whether a reduction in the level of Foxe3 protein is the cause of the phenotype. In the Foxe3 null strain that we have generated, the heterozygous animals are normal during embryonic development, but many of them display keratolenticular adhesion in adults. This provides strong evidence that a reduction of the Foxe3 protein by 50% is causing the abnormal lens phenotype. In summary, our results indicate that Foxe3 has a dual role in lens formation and that this gene might regulate the proliferation of epithelial cells as well as the transition from the proliferative phase to differentiation. We show that the absence of Foxe3 function leads to a premature cell cycle exit, and although the fiber fate is unchanged, their differentiation does not proceed normally. We believe that this well-defined Foxe3 null strain will be very useful for further analysis of early events taking place during mammalian lens formation.
While Foxe3 is also expressed in a specific region of the brain, we have not observed any abnormalities in brain development of Foxe3−/− embryos. Therefore, this expression might not be of functional significance.
Acknowledgments
We thank Rina Shah for assistance with in situ hybridization, Jian Min Deng for assistance with tissue culture, and Carolyn Zilinski for a critical reading of the manuscript.
Veterinary resources were supported by National Institutes of Health Cancer Center Support Grant CA16672. This research was sponsored by NEI grant EY12505 and EY12163 to M.J.
REFERENCES
- 1.Accili, D., and K. C. Arden. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421-426. [DOI] [PubMed] [Google Scholar]
- 2.Ashery-Padan, R., T. Marquardt, X. Zhou, and P. Gruss. 2000. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14:2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai, F., J. Xi, R. Higashikubo, and U. P. Andley. 2004. A comparative analysis of alphaA- and alphaB-crystallin expression during the cell cycle in primary mouse lens epithelial cultures. Exp. Eye Res. 79:795-805. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, T. J., H. El-Hodiri, L. Zhang, R. Shah, E. H. Mathers, and M. Jamrich. 2004. Regulation of vertebrate eye development by Rx genes. Int. J. Dev. Biol. 48:761-770. [DOI] [PubMed] [Google Scholar]
- 5.Blixt, A., M. Mahlapuu, M. Aitola, M. Pelto-Huikko, S. Enerback, and P. Carlsson. 2000. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14:245-254. [PMC free article] [PubMed] [Google Scholar]
- 6.Bourguignon, C., J. Li, and N. Papalopulu. 1998. XBF-1, a winged helix transcription factor with dual activity, has a role in positioning neurogenesis in Xenopus competent ectoderm. Development 125:4889-4900. [DOI] [PubMed] [Google Scholar]
- 7.Brissette, J. L., J. Li, J. Kamimura, D. Lee, and G. P. Dotto. 1996. The product of the mouse nude locus, Whn, regulates the balance between epithelial cell growth and differentiation. Genes Dev. 10:2212-2221. [DOI] [PubMed] [Google Scholar]
- 8.Brownell, I., M. Dirksen, and M. Jamrich. 2000. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis 27:81-93. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson, P., and M. Mahlapuu. 2002. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250:1. [DOI] [PubMed] [Google Scholar]
- 10.Chow, R. L., and R. A. Lang. 2001. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 17:255-296. [DOI] [PubMed] [Google Scholar]
- 11.Crisponi, L., M. Deiana, A. Loi, F. Chiappe, M. Uda, P. Amati, L. Bisceglia, L. Zelante, R. Nagaraja, S. Porcu, M. S. Ristaldi, R. Marzella, M. Rocchi, M. Nicolino, A. Lienhardt-Roussie, A. Nivelon, A. Verloes, D. Schlessinger, P. Gasparini, D. Bonneau, A. Cao, and G. Pilia. 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27:159-166. [DOI] [PubMed] [Google Scholar]
- 12.Cvekl, A., and E. R. Tamm. 2004. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays 26:374-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Baere, E., M. J. Dixon, K. W. Small, E. W. Jabs, B. P. Leroy, K. Devriendt, Y. Gillerot, G. Mortier, F. Meire, L. Van Maldergem, W. Courtens, H. Hjalgrim, S. Huang, I. Liebaers, N. Van Regemorter, P. Touraine, V. Praphanphoj, A. Verloes, N. Udar, V. Yellore, M. Chalukya, S. Yelchits, A. De Paepe, F. Kuttenn, M. Fellous, R. Veitia, and L. Messiaen. 2001. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype-phenotype correlation. Hum. Mol. Genet. 10:1591-1600. [DOI] [PubMed] [Google Scholar]
- 14.Dimanlig, P. V., S. C. Faber, W. Auerbach, H. P. Makarenkova, and R. A. Lang. 2001. The upstream ectoderm enhancer in Pax6 has an important role in lens induction. Development 128:4415-4424. [DOI] [PubMed] [Google Scholar]
- 15.Dirksen, M. L., and M. Jamrich. 1995. Differential expression of fork head genes during early Xenopus and zebrafish development. Dev. Genet. 17:107-116. [DOI] [PubMed] [Google Scholar]
- 16.Dirksen, M. L., and M. Jamrich. 1992. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 6:599-608. [DOI] [PubMed] [Google Scholar]
- 17.Dyer, M. A. 2003. Regulation of proliferation, cell fate specification and differentiation by the homeodomain proteins Prox1, Six3, and Chx10 in the developing retina. Cell Cycle 2:350-357. [PubMed] [Google Scholar]
- 18.El-Hodiri, H., N. Bhatia-Dey, K. Kenyon, K. Ault, M. Dirksen, and M. Jamrich. 2001. Fox (forkhead) genes are involved in the dorso-ventral patterning of the Xenopus mesoderm. Int. J. Dev. Biol 45:265-271. [PubMed] [Google Scholar]
- 19.Erickson, R. P. 2001. Forkhead genes and human disease. J. Appl. Genet. 42:211-221. [PubMed] [Google Scholar]
- 20.Fujiwara, M., T. Uchida, N. Osumi-Yamashita, and K. Eto. 1994. Uchida rat (rSey): a new mutant rat with craniofacial abnormalities resembling those of the mouse Sey mutant. Differentiation 57:31-38. [DOI] [PubMed] [Google Scholar]
- 21.Hatini, V., W. Tao, and E. Lai. 1994. Expression of winged helix genes, BF-1 and BF-2, define adjacent domains within the developing forebrain and retina. J. Neurobiol. 25:1293-1309. [DOI] [PubMed] [Google Scholar]
- 22.Kaestner, K. H., W. Knochel, and D. E. Martinez. 2000. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 14:142-146. [PubMed] [Google Scholar]
- 23.Kaufmann, E., and W. Knochel. 1996. Five years on the wings of fork head. Mech. Dev. 57:3-20. [DOI] [PubMed] [Google Scholar]
- 24.Kenyon, K. L., S. A. Moody, and M. Jamrich. 1999. A novel fork head gene mediates early steps during Xenopus lens formation. Development 126:5107-5116. [DOI] [PubMed] [Google Scholar]
- 25.Kidson, S. H., T. Kume, K. Deng, V. Winfrey, and B. L. Hogan. 1999. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev. Biol. 211:306-322. [DOI] [PubMed] [Google Scholar]
- 26.Lai, C. S., S. E. Fisher, J. A. Hurst, F. Vargha-Khadem, and A. P. Monaco. 2001. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413:519-523. [DOI] [PubMed] [Google Scholar]
- 27.Lai, E., V. R. Prezioso, E. Smith, O. Litvin, R. H. Costa, and J. E. Darnell, Jr. 1990. HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 4:1427-1436. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann, O. J., J. C. Sowden, P. Carlsson, T. Jordan, and S. S. Bhattacharya. 2003. Fox's in development and disease. Trends Genet. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 29.Li, J., and P. K. Vogt. 1993. The retroviral oncogene qin belongs to the transcription factor family that includes the homeotic gene fork head. Proc. Natl. Acad. Sci. USA 90:4490-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, S., Z. Mo, X. Yang, S. M. Price, M. M. Shen, and M. Xiang. 2004. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron 43:795-807. [DOI] [PubMed] [Google Scholar]
- 31.McMahon, A. P., and A. Bradley. 1990. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62:1073-1085. [DOI] [PubMed] [Google Scholar]
- 32.Morrison-Graham, K., G. C. Schatteman, T. Bork, D. F. Bowen-Pope, and J. A. Weston. 1992. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development 115:133-142. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto, S., K. Kawane, R. Watanabe-Fukunaga, H. Fukuyama, Y. Ohsawa, Y. Uchiyama, N. Hashida, N. Ohguro, Y. Tano, T. Morimoto, Y. Fukuda, and S. Nagata. 2003. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature 424:1071-1074. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura, D. Y., R. E. Swiderski, W. L. Alward, C. C. Searby, S. R. Patil, S. R. Bennet, A. B. Kanis, J. M. Gastier, E. M. Stone, and V. C. Sheffield. 1998. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat. Genet. 19:140-147. [DOI] [PubMed] [Google Scholar]
- 35.Ormestad, M., A. Blixt, A. Churchill, T. Martinsson, S. Enerback, and P. Carlsson. 2002. Foxe3 haploinsufficiency in mice: a model for Peters' anomaly. Investig. Ophthalmol. Vis. Sci. 43:1350-1357. [PubMed] [Google Scholar]
- 36.Orr-Urtreger, A., and P. Lonai. 1992. Platelet-derived growth factor-A and its receptor are expressed in separate, but adjacent cell layers of the mouse embryo. Development 115:1045-1058. [DOI] [PubMed] [Google Scholar]
- 37.Peters, A. 1906. Uber angeboren Defektbildung der Descementschen Membran. Klin. Mbl. Augenheilk. 44:27-40. [Google Scholar]
- 38.Prowse, D. M., D. Lee, L. Weiner, N. Jiang, C. M. Magro, H. P. Baden, and J. L. Brissette. 1999. Ectopic expression of the nude gene induces hyperproliferation and defects in differentiation: implications for the self-renewal of cutaneous epithelia. Dev. Biol. 212:54-67. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal, S., and R. K. Hawkins. 1979. Dysgenetic lens (dyl)-a new gene in the mouse. Investig. Ophthalmol. Vis. Sci. 18:642-645. [PubMed] [Google Scholar]
- 40.Sanyal, S., R. Van Nie, J. De Moes, and R. K. Hawkins. 1986. Map position of dysgenetic lens (dyl) locus on chromosome 4 in the mouse. Genet. Res. 48:199-200. [DOI] [PubMed] [Google Scholar]
- 41.Seidman, J. G., and C. Seidman. 2002. Transcription factor haploinsufficiency: when half a loaf is not enough. J. Clin. Investig. 109:451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semina, E. V., I. Brownell, H. A. Mintz-Hittner, J. C. Murray, and M. Jamrich. 2001. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 10:231-236. [DOI] [PubMed] [Google Scholar]
- 43.Smith, G. M., and C. M. Velzeboer. 1975. Peter's anomaly. Ophthalmologica 171:318-320. [DOI] [PubMed] [Google Scholar]
- 44.Smith, R. S., A. Zabaleta, T. Kume, O. V. Savinova, S. H. Kidson, J. E. Martin, D. Y. Nishimura, W. L. Alward, B. L. Hogan, and S. W. John. 2000. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum. Mol. Genet. 9:1021-1032. [DOI] [PubMed] [Google Scholar]
- 45.Spemann, H. 1901. Ueber Korrelationen in der Entwicklung des Auges, vol. 15. Jena Verlag, Bonn, Germany.
- 46.Tseng, H. T., R. Shah, and M. Jamrich. 2004. Function and regulation of FoxF1 during Xenopus gut development. Development 131:3637-3647. [DOI] [PubMed] [Google Scholar]
- 47.Weigel, D., and H. Jackle. 1990. The fork head domain: a novel DNA binding motif of eukaryotic transcription factors? Cell 63:455-456. [DOI] [PubMed] [Google Scholar]
- 48.Wigle, J. T., K. Chowdhury, P. Gruss, and G. Oliver. 1999. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21:318-322. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson, D. G. 1992. Whole mount in situ hybridization of vertebrate embryos. Oxford University Press, Oxford.
- 50.Xuan, S., C. A. Baptista, G. Balas, W. Tao, V. C. Soares, and E. Lai. 1995. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14:1141-1152. [DOI] [PubMed] [Google Scholar]
- 50a.Yuasa, J., S. Hirano, M. Yamagata, and M. Noda. Visual projection map specified by topographic expression of transcription factors in the retina. Nature 382:632-635. [DOI] [PubMed]
- 51.Zhang, P., C. Wong, R. A. DePinho, J. W. Harper, and S. J. Elledge. 1998. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 12:3162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zilinski, C., I. Brownell, R. Hashimoto, O. Medina-Martinez, E. Swindell, and M. Jamrich. 2004. Expression of FoxE4 and Rx genes visualizes the timing and dynamics of critical processes taking place during initial stages of vertebrate eye development. Dev. Neurosci. 26:1-14. [DOI] [PubMed] [Google Scholar]