Abstract
The mechanism of cross talk between the Wnt signaling and cyclic AMP (cAMP)-dependent protein kinase (protein kinase A [PKA]) pathways was studied. Prostaglandin E1 (PGE1), isoproterenol, and dibutyryl cAMP (Bt2cAMP), all of which activate PKA, increased the cytoplasmic and nuclear β-catenin protein level, and these actions were suppressed by a PKA inhibitor and RNA interference for PKA. PGE1 and Bt2cAMP also increased T-cell factor (Tcf)-dependent transcription through β-catenin. Bt2cAMP suppressed degradation of β-catenin at the protein level. Although PKA did not affect the formation of a complex between glycogen synthase kinase 3β (GSK-3β), β-catenin, and Axin, phosphorylation of β-catenin by PKA inhibited ubiquitination of β-catenin in intact cells and in vitro. Ser675 was found to be a site for phosphorylation by PKA, and substitution of this serine residue with alanine in β-catenin attenuated inhibition of the ubiquitination of β-catenin by PKA, PKA-induced stabilization of β-catenin, and PKA-dependent activation of Tcf. These results indicate that PKA inhibits the ubiquitination of β-catenin by phosphorylating β-catenin, thereby causing β-catenin to accumulate and the Wnt signaling pathway to be activated.
β-Catenin not only regulates cell-to-cell adhesion by interacting with cadherin but also functions as a component of the Wnt signaling pathway (34). The Wnt signaling pathway is conserved evolutionally and regulates cellular proliferation and differentiation by stabilizing β-catenin (2, 37). The β-catenin gene is often mutated in human cancer, and in such cases the protein level of β-catenin increases (17, 35). Therefore, clarifying the regulation of β-catenin stabilization is important for understanding the molecular mechanism of tumor formation.
According to the most widely accepted current model, casein kinase Iα (CKIα) and glycogen synthase kinase 3β (GSK-3β) target cytoplasmic β-catenin for degradation in the absence of Wnt (13, 26, 42). Axin has been shown to form a complex with GSK-3β, CKIα, β-catenin, and adenomatous polyposis coli gene product (APC) (13, 16, 21, 26). In the Axin complex CKIα serves as a priming kinase that phosphorylates Ser45 of β-catenin and enhances the phosphorylation at Ser33, Ser37, and Thr41 of β-catenin by GSK-3β (26, 43). Phosphorylated β-catenin is ubiquitinated and degraded by the proteasome pathway (22).
When Wnt acts on its cell surface receptor consisting of Frizzled and lipoprotein receptor-related protein (LRP) 5/6 (9), Dvl induces the accumulation of β-catenin in the cytoplasm by inhibiting the GSK-3β-dependent phosphorylation of β-catenin (10, 20, 41). Accumulated β-catenin is translocated into the nucleus, where it binds to the transcription factors T-cell factor (Tcf) and lymphoid enhancer factor (Lef) and thereby stimulates the expression of various genes, including c-myc, cyclin D1, and Axin2 (2, 28, 37). Thus, Wnt stabilizes β-catenin and activates Tcf and Lef. Although CKIα- and GSK-3β-dependent phosphorylation is essential for the degradation of β-catenin in the Wnt pathway, a phosphorylation-independent pathway through Siah-1 has been found (27, 30). It has also been shown that release of Ca2+ from internal stores by the Gq pathway results in calpain-mediated degradation of β-catenin (25). Therefore, it is likely that there are multiple pathways to regulate the stability of β-catenin.
The Alzheimer's disease-linked gene presenilin1 forms a complex with GSK-3β, β-catenin, and the catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase (PKA) (15). In the presenilin1 complex, PKA phosphorylates Ser45 of β-catenin and enhances the GSK-3β-dependent phosphorylation of β-catenin, suggesting that PKA and presenilin1 induce the downregulation of β-catenin independently of the Wnt-controlled Axin complex. Indeed, nuclear accumulation of β-catenin is observed in the epidermis of presenilin1-deficient mice (15). However, in contrast to the finding that PKA might function as a negative regulator of β-catenin stability, it has been shown that stimulation with prostaglandin E2 (PGE2), which activates PKA, increases the transcriptional activity of Tcf in HEK-293 cells, probably through phosphorylation and inhibition of GSK-3β (8). Thus, the molecular mechanism of the cross talk between the Wnt and PKA signaling pathways is largely unknown. In this study, we examined the effects of PKA on the ubiquitin-dependent degradation of β-catenin. We here show that phosphorylation of β-catenin at Ser675 by PKA stabilizes β-catenin by inhibiting its ubiquitination.
MATERIALS AND METHODS
Materials and chemicals.
pRSETB/human CKI α and CKI ɛ; pCGN/ubiquitin; pcDNA3/ β2AR; Axin2-luciferase (Axin2-Luc); and pcDNAI/hTcf-4E, Top-fos -Luc, and Fop-fos -Luc were kindly provided by D. M. Virshup (University of Utah, Salt Lake City), M. Nakao (Kumamoto University, Kumamoto, Japan), R. Lefkovitz (Duke University, Durham, NC), F. Costantini (Columbia University, New York, N.Y.), and H. Clevers (University Medical Center, Utrecht, The Netherlands), respectively. The anti-Axin antibody was prepared in rabbits as described previously ( 41 ). The rabbit polyclonal antibody against β-catenin phosphorylated at Ser675 (anti-pS675 antibody) was raised using the phosphopeptide Cys-Tyr-Lys-Lys Arg-Leu-phospho-Ser675-Val-Glu-Leu-Thr-Ser for β-catenin as the antigen (Peptide Institute, Inc., Minoh, Japan). The antiserum obtained was then affinity purified against the respective phosphopeptide. L cells stably expressing β2-adrenergic receptor (β2AR) were produced by transfecting pcDNA3/ β2AR and pNeo. Wnt-3a-conditioned medium was prepared as described previously (1, 9). Myc-Uba1-His6 and His6-hUbc5a-FLAG were purified from Escherichia coli according to the supplier's instructions. The recombinant SCFSkp1 complex was purified using TALON metal affinity resin (Clontech) from lysates of Sf9 cells that had been coinfected with baculovirus encoding His6-T7-Skp1, HA-Cul1, glutathione S-transferase (GST)-Myc-Fbw1, and Myc-Rbx1. The anti-GSK-3β and anti-catalytic subunit of PKA (PKAc), anti-β-catenin and anti-phospho-β-catenin (Ser33/Ser37/Thr41 or Thr41/Ser45), and anti-FLAG antibodies were purchased from BD Transduction Laboratories, Cell Signaling Technology, and Sigma, respectively. The anti-α-tubulin, anti-histone H1, anti-IκBα, and anti-APC (Ab-1) antibodies were from Molecular Probes, Upstate Biotechnology, Santa Cruz Biotechnology, and Calbiochem, respectively. Other materials and chemicals were from commercial sources.
Plasmid construction.
pGEX-2T/GSK-3β, pMAL-c2/rAxin, pGEX-2T/β-catenin, pRSETA/β-catenin, pCGN/β-catenin, pBJ-Myc/rAxin, pBacPAK9/Myc-Fbw1, pBacPAK8/HA-Cul1, pBacPAK8/His6-T7-Skp1, pBacPAK8/Myc-Rbx1, pET23B/Myc-Uba1-His6, pRSETB/hUbc5a-FLAG, pEF-BOS-HA/hTcf-4E, and pEF-BOS-HA/hTcf-4E-(Δ1-53) were constructed as described previously (13, 14, 19, 21, 22, 32, 40). pMAL-c2/hCKIα, pCGN/β-cateninSA, pCGN/β-cateninT510A, pCGN/β-cateninS675A, pRSETA/β-cateninT510A, pRSETA/β-cateninS675A, and pCGN/PKAc were made by inserting the respective cDNA fragments generated by PCR into the vector.
Accumulation and phosphorylation of β-catenin by PKA.
To detect the accumulation of β-catenin induced by PKA activation in L cells and HEK-293 cells, the cells (60-mm-diameter dish) were stimulated with 50 ng/ml prostaglandin E1 (PGE1) or 1 mM dibutyryl cyclic AMP (Bt2cAMP) and 0.1 mM 3-isobutyl-1-methylxanthine (IBMX) for 0.5 to 2 h in the presence or absence of 25 μg/ml cycloheximide. After the stimulation, L cells were lysed in 200 μl of lysis buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 20 μg/ml leupeptin, 20 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 25 mM β-glycerophosphate, 5 mM sodium orthovanadate, and 5 mM NaF). HEK-293 cells were suspended in 200 μl of homogenizing buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM dithiothreitol [DTT], 20 μg/ml leupeptin, 20 μg/ml aprotinin, 1 mM PMSF, 25 mM β-glycerophosphate, and 5 mM NaF). This suspension was sonicated on ice, and the homogenate was centrifuged at 100,000 × g for 30 min at 4°C. The supernatant was used for the experiments. When necessary, L cells and HEK-293 cells were stimulated with Wnt-3a-conditioned medium. The lysates of L cells and the supernatants of HEK-293 cells were probed with the anti-β-catenin, anti-GSK-3β, or anti-phospho-β-catenin (Ser33/Ser37/Thr41 or Thr41/Ser45) antibody. Since the antigen recognition specificity of the anti-phospho-β-catenin (Thr41/Ser45) antibody did not overlap that of the anti-phospho-β-catenin (Ser33/Ser37/Thr41) antibody (15), this antibody was designated as an anti-P-45 β-catenin antibody in this study.
RNA interference.
To knock down the catalytic subunits (α and β) of PKA, double-stranded RNA (dsRNA) oligonucleotides were synthesized by in vitro transcription of RNA from small DNA templates containing T7 promoter sequences, using a CUGA7 in vitro small interfering RNA synthesis kit (Nippon Gene, Tokyo, Japan). The targeted sequences of mouse PKAcα and PKAcβ were 5′-AAGTGGTTTGCCACGACTGAC-3′ and 5′-AAAGAGTTTCTAGCCAAAGCC-3′, respectively. Transfection of dsRNA oligonucleotides was done as described previously (10).
Quantitative RT-PCR.
Total RNA was isolated from HEK-293 cells after they had been treated with 1 mM Bt2cAMP and 0.1 mM IBMX or Wnt-3a-conditioned medium for 2 h. Each RNA sample (5 μg) was subjected to reverse transcription using murine leukemia virus reverse transcriptase (PE Applied Biosystems) in a total volume of 20 μl. Quantitative reverse transcription-PCR (RT-PCR) was performed using a LightCycler (Roche Molecular Biochemicals). Aliquots (0.5 μl) of the reverse transcription products were amplified in a reaction mixture (20 μl) containing LightCycler FastStart DNA Master SYBR Green I, 0.5 μM primer, and 5 mM MgCl2 for β-catenin, 4 mM MgCl2 for Axin2, or 3 mM MgCl2 for cyclin D1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Forward and reverse primers were as follows: β-catenin, 5′-TTCGCCTTCACTATGGAC-3′ and 5′-GTGGAATGGCACCCTG-3′; cyclin D1, 5′-CATCAAGTGTGACCCGGACTG-3′ and 5′-CCTCCTCCTCAGTGGCCTTG-3′; Axin2, 5′-GTGAGTTGGTTGTCACTTAC-3′ and 5′-GTTTTGTCGCAGTTGCT-3′; GAPDH, 5′-CCTGTTCGACAGTCAGCCG-3′ and 5′-CGACCAAATCCGTTGACTCC-3′.
Phosphorylation of β-catenin by GSK-3β, PKA, and CKIα in vitro.
His6-β-catenin (2 μg of protein) in the presence or absence of maltose-binding protein (MBP)-rAxin (400 ng of protein) was incubated with 20 U of PKAc (Promega) or 0.125 μM MBP-CKIα in 20 μl of kinase reaction mixture (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 mM DTT) containing 50 μM ATP for 30 min at 30°C, followed by further incubation with 0.1 μM GST-GSK-3β in 30 μl of kinase reaction mixture containing 50 μM [γ-32P]ATP (500 to 1,000 cpm/pmol) for 30 min at 30°C. Aliquots of the samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by autoradiography and Western blotting.
In vitro ubiquitination assay.
Purified His6-β-catenin (4 μg of protein), MBP-rAxin (1 μg of protein), His6-CΚIɛ (200 ng of protein), and GST GSK-3β (200 ng of protein) were incubated in 11 μl of kinase reaction mixture containing 50 μM ATP for 2 h at 30°C. After the incubation, the mixtures were incubated with 3 μg of His6-Myc-ubiquitin, 100 ng of Myc-Uba1-His6, 500 ng of His6-hUbc5a-FLAG, and 200 ng of recombinant SCFSkp1 complex in 38 μl of ubiquitination reaction mixture (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 2 mM DTT, 2.5 mM ATP, 1 mM phosphocreatine, 50 U/ml phosphocreatine kinase, 2 μM okadaic acid, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF) for 1 h at 30°C. The samples were immunoprecipitated with the anti-β-catenin antibody. The immunoprecipitates were probed with the anti-β-catenin antibody.
To examine the effects of PKA on the ubiquitination of β-catenin in vitro, some proteins were preincubated with PKA before the ubiquitination assay. For example, 4 μg of His6-β-catenin or MBP-rAxin was incubated with 40 U of PKAc in 5 μl of kinase reaction mixture containing 50 μM ATP in the presence or absence of 40 μM N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89) for 30 min at 30°C. Recombinant SCF complex (200 ng of proteins) was incubated with 4 U of PKAc in 15 μl of kinase reaction mixture containing 50 μM ATP for 1 h at 30°C. After the preincubation, 40 μM H-89 was added to inhibit PKAc.
Pulse-chase analysis of β-catenin.
COS cells (60 to 70% confluent on a 35-mm-diameter dish) were transfected with pCGN/β-catenin, pCGN/β-cateninS675A, or pCGN/β-cateninT510A. After 48 h, cells were preincubated for 1 h in Dulbecco's modified Eagle's medium lacking the amino acids methionine and cystine (GIBCO), followed by incubation with [35S]methionine and [35S]cysteine (50 μCi/ml) for 30 min at 37°C (21). Then the cells were lysed immediately or at the indicated times following incubation with excess unlabeled methionine and cysteine in the presence or absence of 1 mM Bt2cAMP and 0.1 mM IBMX. The lysates were immunoprecipitated with the antihemagglutinin (anti-HA) antibody, and the precipitates were probed with the anti-β-catenin antibody and analyzed with the Molecular Dynamics Storm imaging system.
Other assays.
Tcf-4 transcriptional activity was measured as described previously (40). The GSK-3β activity was assayed by the use of synthetic peptides as substrates (13, 32, 38). Immunoblotting, immunoprecipitation, and subcellular fractionation were performed as described previously (10-12, 18).
RESULTS
Accumulation of β-catenin as a result of activation of PKA.
We first examined whether PKA stimulators affect the protein level of β-catenin. Wnt-3a-conditioned medium stabilized β-catenin in mouse fibroblast L cells (Fig. 1A). PGE1, which activates PKA in L cells (4), also induced the accumulation of β-catenin (Fig. 1A). H-89, a PKA inhibitor, completely suppressed the increase in the protein level of β-catenin induced by PGE1 but did not inhibit that induced by Wnt-3a (Fig. 1A). Since isoproterenol, a β-agonist, did not affect the protein level of β-catenin in wild-type L cells, we generated L cells stably expressing β2-adrenergic receptor (L/β2AR cells). Isoproterenol increased the level in L/β2AR cells, and isoproterenol-induced accumulation of β-catenin was inhibited by H-89 (Fig. 1B). Treatment of HEK-293 cells with Bt2cAMP, a cell-permeable cyclic AMP analogue, induced the accumulation of β-catenin (Fig. 1C). Although IBMX, a phosphodiesterase inhibitor, itself did not affect the β-catenin protein level, it enhanced the Bt2cAMP-induced accumulation of β-catenin (Fig. 1C). Forskolin, which is an activator of adenylate cyclase, also induced the accumulation of β-catenin, and the action of Bt2cAMP was inhibited by H-89 (Fig. 1C). 12-O-Tetradecanoylphorbol 13-acetate (TPA), which activates protein kinase C, did not affect the cytosolic β-catenin level (Fig. 1C). Further, dsRNA oligonucleotides for PKAc suppressed Bt2cAMP-induced accumulation of β-catenin (Fig. 1D). These results indicate that activation of PKA results in the accumulation of β-catenin in these cell lines. Since the combination of Bt2cAMP and IBMX clearly induces the accumulation of β-catenin, we used these reagents further in this study. Bt2cAMP increased β-catenin in both the fractions of the cytoplasm and of the nucleus in L cells as well as Wnt-3a did (Fig. 1E). Wnt-3a and Bt2cAMP additively induced β-catenin accumulation in HEK-293 and L cells (Fig. 1F), suggesting that Wnt-3a and PKA regulate the protein level of β-catenin through different mechanisms.
FIG. 1.
Accumulation of β-catenin induced by PKA activation in intact cells. (A) After L cells were preincubated with or without 10 μM H-89 for 30 min, the cells were stimulated with 50 ng/ml PGE1 or Wnt-3a-conditioned medium for 2 h. The lysates were probed with the indicated antibodies. Ab, antibody. (B) Wild-type L cells (L/WT) and L cells stably expressing β2AR (L/β2AR) were treated with 10 μM isoproterenol in the presence or absence of H-89 for 2 h. The lysates were probed with the indicated antibodies. (C) HEK-293 cells were stimulated with 1 mM Bt2cAMP and/or 0.1 mM IBMX for 2 h (lanes 1 to 4). Before HEK-293 cells were stimulated with the indicated reagents, the cells were preincubated with or without 10 μM H-89 for 30 min (lanes 5 to 10). The cytosol was probed with the indicated antibodies. (D) RNA interference for PKAc. L cells were transfected with dsRNA oligonucleotides for PKAcα and PKAcβ. The cells were stimulated with Bt2cAMP and IBMX for 2 h, and the lysates were probed with the indicated antibodies. (E) The cytoplasm and nuclear fractions from L cells stimulated with Bt2cAMP and IBMX or Wnt-3a for 2 h were probed with the indicated antibodies. (F) HEK-293 or L cells were stimulated with Bt2cAMP and IBMX, Wnt-3a, or both for 2 h. The cytosol of HEK-293 cells or the lysate of L cells was probed with the indicated antibodies. The results shown are representative of three independent experiments.
Activation of Tcf-4 by PKA through β-catenin.
To examine the physiological relevance of PKA-dependent accumulation of β-catenin, we measured Tcf-4 transcriptional activity using TOP-fos-Luc (23), which contains four Tcf-binding sites and a c-fos promoter sequence ligated to a luciferase gene, as a reporter plasmid. Treatment of L cells with PGE1, Bt2cAMP, or Wnt-3a activated Tcf-4 (Fig. 2A). PGE1 or Bt2cAMP activated Tcf-4-(Δ1-53), in which the β-catenin-binding site is deleted, to a lesser degree compared with wild-type Tcf-4, and Wnt-3a did not activate Tcf-4-(Δ1-53) (Fig. 2A). When FOP-fos-Luc, in which the Tcf-binding sites were mutated, was used instead of TOP-fos-Luc, Wnt-3a-dependent Tcf-4 activity was completely abolished, while PGE1- and Bt2cAMP-dependent Tcf-4 activity decreased to about 40 to 50% of the control level (Fig. 2A). These results suggest that PKA is able to activate Tcf-4 through β-catenin. However, there was no difference between the activation of wild-type Tcf-4 and that of Tcf-4-(Δ1-53) by PGE1 or Bt2cAMP. The reason why PGE1 or Bt2cAMP still triggers Tcf-4 activity toward FOP-fos-Luc would be that the fos promoter in FOP-fos-Luc contains a cAMP-responsive element (CRE) that interacts with CRE-binding protein (CREB) phosphorylated by PKA.
FIG. 2.
Activation of Tcf-4 by PKA. (A) Activation of Tcf-4 by PGE1 and Bt2cAMP. L cells transfected with pEF-BOS-HA/hTcf-4E (0.1 μg) and TOP-fos-Luc (0.5 μg) or FOP-fos-Luc (0.5 μg) were treated with PGE1, Bt2cAMP and IBMX, or Wnt-3a for 8 h. The luciferase activity was measured and expressed as the fold increase compared with the level observed in cells without treatment. (B) Effects of PKA on the Tcf-4 activity toward a natural Tcf-responsive promoter. pEF-BOS-HA/hTcf-4E (0.1 μg) or pEF-BOS-HA/hTcf-4E (Δ1-53) (0.1 μg) was transfected into L cells with Axin2-Luc (0.5 μg). The cells were treated with PGE1, Bt2cAMP and IBMX, or Wnt-3a in the presence or absence of H-89 for 8 h. The luciferase activity was measured and expressed as the fold increase compared with the level observed in the cells without treatment. (C) Effects of PKA on expression of the Axin2 mRNA. Total RNA from HEK-293 cells stimulated with Bt2cAMP and IBMX or Wnt-3a for 2 h in the presence or absence of H-89 was subjected to quantitative RT-PCR. The results shown were normalized by the GAPDH mRNA levels, and the corresponding quantification results for the Axin2 mRNA levels were expressed as the fold increase compared with that of cells without treatment. The results shown are means ± standard errors from four independent experiments.
Since TOP-fos-Luc is a synthetic promoter, we also tested the natural promoter of Axin2 (5.6-kb DNA that contains the mouse Axin2 promoter, exon1, and intron1) ligated to a luciferase gene (Axin2-Luc), because the expression of the Axin2 gene is stimulated by β-catenin and Tcf (28). PGE1 or Bt2cAMP activated Tcf-4 but not Tcf-4-(Δ1-53) activity toward the Axin2 promoter as well as Wnt-3a (Fig. 2B). H-89 almost completely inhibited PGE1- or Bt2cAMP-induced Tcf-4 activation but did not inhibit Wnt-3a-dependent activation (Fig. 2B). Consistent with these results, quantitative RT-PCR analyses also showed that Bt2cAMP increases the Axin2 mRNA in HEK-293 cells and that H-89 represses this elevation (Fig. 2C). Wnt-3a also increased the mRNA level of Axin2, whereas H-89 did not affect the elevation (Fig. 2C). Since typical CRE (TGACGTCA) (31) is not observed in the human Axin2 genomic sequence that corresponds to the 5.6-kb DNA of mouse Axin2 promoter and intron1, these results indicate that PKA is able to induce the expression of the Axin2 gene in a manner dependent on β-catenin.
Inhibition of degradation of β-catenin by PKA.
Neither Bt2cAMP nor Wnt-3a affected the mRNA level of β-catenin under the conditions in which Bt2cAMP and Wnt-3a increased the mRNA level of cyclin D1 in HEK-293 cells (Fig. 3A). Therefore, we examined the effects of PKA on the degradation of β-catenin at the protein level. When L cells were treated with cycloheximide, which inhibits protein synthesis, the β-catenin protein almost disappeared by 60 min. Bt2cAMP inhibited the degradation of β-catenin as well as Wnt-3a (Fig. 3B). IκBα is degraded by the proteasome pathway in response to tumor necrosis factor alpha (TNF-α) (3). Treatment of HEK-293 cells with Bt2cAMP did not inhibit TNF-α-dependent degradation of IκBα but rather slightly enhanced it (Fig. 3C), consistent with previously reported observations (1). Therefore, PKA-dependent β-catenin accumulation may be specific. These results demonstrate that PKA does not regulate the expression of the β-catenin gene but affects the posttranslational stabilization of β-catenin.
FIG. 3.
Inhibition of degradation of β-catenin by PKA. (A) Effects of PKA on expression of the β-catenin mRNA. HEK-293 cells were treated with Bt2cAMP and IBMX or Wnt-3a for 2 h and then subjected to quantitative RT-PCR. The results shown were normalized using the GAPDH mRNA levels, and the corresponding quantification results for cyclin D1 and β-catenin mRNA levels were expressed as the fold increase compared with those in the absence of Bt2cAMP and Wnt-3a. The results shown are means ± standard errors from four independent experiments. (B) Effects of PKA on protein degradation of β-catenin. L cells were treated with Bt2cAMP and IBMX or Wnt-3a for the periods indicated in the presence of 25 μg/ml cycloheximide. The β-catenin protein levels were normalized using the GSK-3β protein level and expressed as arbitrary units. (C) Effects of PKA on the protein levels of IκBα. HEK-293 cells were preincubated with or without Bt2cAMP and IBMX for 1 h and then stimulated with 10 ng/ml TNF-α for 10 min. The cytosol was probed with the indicated antibodies. The results shown are representative of three independent experiments.
Effects of PKA on the complex formation of Axin, GSK-3β, β-catenin, and APC.
PKA has been shown to phosphorylate APC, GSK-3β, and β-catenin (7, 15, 36). In addition, PKAc phosphorylated Axin but not CKIα in vitro (data not shown). When fully phosphorylated, about 2, 0.5, 2, and 1 mol of phosphate were incorporated into 1 mol of APC, GSK-3β, β-catenin, and Axin, respectively. It is known that Axin binds to GSK-3β, APC, and β-catenin to degrade β-catenin (17, 35). When Axin was immunoprecipitated from L cells with the anti-Axin antibody, it formed a complex with GSK-3β, β-catenin, and APC at the endogenous levels (Fig. 4A). Treatment of L cells with Bt2cAMP did not affect the binding of Axin to GSK-3β, β-catenin, and APC (Fig. 4A). Direct phosphorylation of GST-β-catenin or GST-GSK-3β by PKAc did not affect their binding to MBP-rAxin in vitro (data not shown). It has been suggested that Wnt-dependent downregulation of Axin is one of the mechanisms to stabilize β-catenin (39, 41). However, Bt2cAMP did not induce the downregulation of Axin in L cells under the conditions in which Wnt-3a did (Fig. 4B). Therefore, PKA does not affect the formation of a complex between Axin, GSK-3β, β-catenin, and APC.
FIG. 4.
(A) Effects of PKA on the complex formation of Axin, β-catenin, GSK-3β, and APC. After L cells were incubated with Bt2cAMP and IBMX for 2 h, the lysates were probed with the indicated antibodies. The lysates were immunoprecipitated with the anti-Axin antibody, and the immunoprecipitates were probed with the indicated antibodies. IP, immunoprecipitation. (B) Effects of PKA on the stability of Axin. L cells were treated with Bt2cAMP and IBMX or Wnt-3a for the periods indicated. The lysates were probed with the indicated antibodies.
Effects of PKA on GSK-3β activity in the Axin complex.
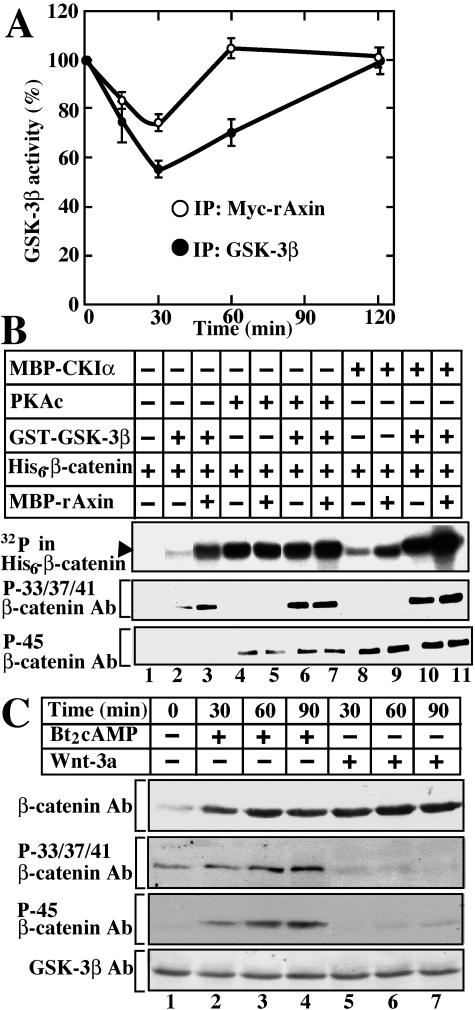
Since it has been shown that PKA phosphorylates Ser9 of GSK-3β and inhibits its activity (7), we tested the effects of PKA on the activity of GSK-3β bound to Axin. L cells stably expressing Myc-rAxin were treated with Bt2cAMP to activate PKA, and the lysates were immunoprecipitated with the anti-GSK-3β antibody. The total GSK-3β activity decreased by 55% at 30 min after stimulation, and the activity remained low at 60 min (Fig. 5A). When the same lysates were immunoprecipitated with the anti-Myc antibody, the activity of GSK-3β in the Myc-rAxin complex decreased less remarkably than that of total GSK-3β (Fig. 5A). By 60 min, the GSK-3β activity had completely recovered. These results suggest that it is hard for PKA to act on GSK-3β complexed with Axin. The slight inhibition of the GSK-3β activity in the Axin complex by PKA may lower the phosphorylation state of β-catenin. However, it is also possible that PKA stabilizes β-catenin at a step other than inhibition of GSK-3β.
FIG. 5.
Phosphorylation of GSK-3β and β-catenin by PKA. (A) Regulation of GSK-3β activity by PKA in the Axin complex. After L cells stably expressing Myc-rAxin were treated with Bt2cAMP and IBMX for the periods indicated, the activities of total GSK-3β (•) and GSK-3β complexed with Axin (○) were measured. The results shown are expressed as a percentage of the activity of GSK-3β at time zero and are means ± standard errors from three independent experiments. (B) Phosphorylation of β-catenin by PKA, CKIα, and GSK-3β in vitro. His6-β-catenin (2 μg of protein) was incubated with or without 0.1 μM GST-GSK-3β in the presence or absence of MBP-rAxin (400 ng of protein) for 30 min after prephosphorylation by 0.125 μM MBP-CKIα or 20 U of PKAc. The top panel is an autoradiograph that shows the total phosphate incorporated into His6-β-catenin. The phosphorylation states of His6-β-catenin were detected with the indicated antibodies. (C) Phosphorylation of β-catenin by PKA in intact cells. HEK-293 cells were stimulated with Bt2cAMP and IBMX or Wnt-3a for the periods indicated. The cytosol was probed with the indicated antibodies. The results shown are representative of three independent experiments.
Enhancement of GSK-3β-dependent phosphorylation of β-catenin by PKA.
As CKIα and PKA are reported to phosphorylate Ser45 of β-catenin (15, 26, 43), we compared their abilities to function as priming kinases for GSK-3β to phosphorylate Ser33, Ser37, and Thr41 using purified proteins. GSK-3β induced pronounced phosphorylation of Ser33, Ser37, and Thr41 of β-catenin when Ser45 was phosphorylated by CKIα (Fig. 5B, lanes 2, 8, and 10). Axin enhanced CKIα- and GSK-3β-dependent phosphorylation of Ser33, Ser37, and Thr41 of β-catenin (Fig. 5B, lanes 2, 3, and 8 to 11). Detection of the phosphorylation by autoradiography was more sensitive than detection using the anti-phospho-β-catenin antibody. PKAc phosphorylated β-catenin at Ser45, and the level of phosphorylation was higher than that of CKIα-induced phosphorylation when detected by autoradiography (Fig. 5B, lanes 4 and 8), suggesting that PKAc efficiently phosphorylates serine or threonine residues of β-catenin other than Ser45. PKAc also enhanced GSK-3β-dependent phosphorylation of β-catenin at Ser33, Ser37, and Thr41 (Fig. 5B, lanes 2 and 6). However, Axin did not affect PKAc- and GSK-3β-dependent phosphorylation of β-catenin (Fig. 5B, lanes 6 and 7). These results demonstrate that PKA acts as a priming kinase for GSK-3β-induced β-catenin phosphorylation in vitro.
We next examined the phosphorylation state of β-catenin when PKA was activated in intact cells. Consistent with the previous observations (26), Wnt-3a did not affect the phosphorylation of Ser45 but inhibited that of Ser33, Ser37, and Thr41 of β-catenin in HEK-293 cells, resulting in the accumulation of β-catenin (Fig. 5C, lanes 1 and 5 to 7). In contrast, Bt2cAMP increased the level of phosphorylation of Ser45 of β-catenin and enhanced the phosphorylation at Ser33, Ser37, and Thr41 in a time-dependent manner (Fig. 5C, lanes 1 to 4). Irrespective of the enhancement of phosphorylation of β-catenin, β-catenin was stabilized. This result also suggests a decrease in relative phosphorylation of Ser33, Ser37, and Thr41 of β-catenin, because the β-catenin levels were high while the phosphorylation levels increased slightly. Therefore, we cannot exclude the possibility that PKA inhibits GSK-3 in the Axin complex, thereby stabilizing β-catenin. However, taken together with the observations that PKA weakly inhibits GSK-3β complexed with Axin and that PKA phosphorylates β-catenin more efficiently at serine or threonine residues other than Ser45, these results prompted us to examine the possibility that the mechanism of stabilization of β-catenin by PKA is different from that by Wnt-3a.
Inhibition of ubiquitination of β-catenin by PKA.
We examined whether PKA affects the ubiquitination of β-catenin in intact cells. Ubiquitination of various proteins was observed when HA-ubiquitin was expressed in HEK-293 cells in the presence of lactacystin, a proteasome inhibitor, and treatment with Bt2cAMP did not affect the extent of ubiquitination (Fig. 6A, lanes 1 to 3). HA-positive high-molecular-weight proteins were detected in the β-catenin immunoprecipitates, and Bt2cAMP decreased the extent of the formation of HA-positive proteins (Fig. 6A, lanes 4 to 6). Furthermore, the same samples were probed with the anti-β-catenin antibody. Slowly migrating forms, which are known to be the ubiquitinated forms, were observed in the β-catenin immunoprecipitates, and treatment with Bt2cAMP decreased the intensity of the slowly migrating forms (Fig. 6A, lanes 7 to 9). Since the cells were treated with lactacystin in this experiment, Bt2cAMP did not further increase β-catenin (Fig. 6A, lanes 7 to 9). When MG-132 was used instead of lactacystin, similar results were obtained (data not shown).
FIG. 6.
Inhibition of ubiquitination of β-catenin by PKA. (A) Decrease in ubiquitination of β-catenin by PKA in intact cells. HEK-293 cells expressing HA-ubiquitin and FLAG-Fbw1 were incubated with (lanes 3, 6, and 9) or without (lanes 1, 2, 4, 5, 7, and 8) Bt2cAMP and IBMX for 2 h in the presence of 10 μM lactacystin. The lysates were probed with the anti-HA antibody (lanes 1 to 3). After the lysates had been immunoprecipitated with the anti-Myc (lanes 4 and 7) or anti-β-catenin (lanes 5, 6, 8, and 9) antibody, the immunoprecipitates were probed with the anti-HA (lanes 4 to 6) or β-catenin (lanes 7 to 9) antibody. The arrowhead indicates the position of β-catenin. (B) Complex formation of Fbw1 and β-catenin. L cells expressing FLAG-Fbw1 were stimulated with Bt2cAMP and IBMX for 2 h. The lysates were immunoprecipitated with the anti-FLAG antibody. The immunoprecipitates were probed with the indicated antibodies. (C) Effects of Fbw1 on PKA-dependent nuclear localization of β-catenin. HEK-293 cells expressing FLAG-Fbw1 and HA-β-catenin were treated with Bt2cAMP and IBMX for 2 h. The nuclear fractions were prepared and probed with the indicated antibodies. (D) Effects of Fbw1 on PKA-dependent Tcf-activity. pEF-BOS-HA/hTcf-4E (0.1 μg) and Axin2-Luc (0.5 μg) were transfected into L cells with pEF-BOS-FLAG/Fbw1 (0.1 μg). The cells were treated with Bt2cAMP and IBMX, or Wnt-3a, for 8 h. The luciferase activity was measured and expressed as the fold increase compared with the level observed in the cells without treatment. The results shown are means ± standard errors from four independent experiments. (E) The recombinant SCFSkp1 complex was purified from Sf9 cell lysate expressing His6-T7-Skp1, HA-Cul1, GST-Myc-Fbw1, and Myc-Rbx1. The proteins were detected with their antibodies. (F) Decrease in ubiquitination of β-catenin by PKA in vitro. Purified His6-β-catenin was incubated with MBP-rAxin, His6-CΚIɛ, and GST-GSK-3β in the presence (lanes 1 and 2) or absence (lane 3) of ATP in the kinase reaction mixture for 2 h at 30°C. The samples were further subjected to the ubiquitination assay in the presence (lanes 1 and 3) or absence (lane 2) of His6-Myc-ubiquitin for 1 h at 30°C. Before the ubiquitination assay, His6-β-catenin was preincubated with or without PKAc in the presence or absence of H-89 (lanes 4 to 6). (G) Effects of pretreatment of the SCF complex with PKA on ubiquitination of β-catenin. The assay conditions were the same as those in panel F, except that recombinant SCFSkp1 complex pretreated with PKAc for 1 h at 30°C was used. The results shown are representative of three independent experiments.
Fbw1, an F-box protein that is a component of E3 ubiquitin ligase and interacts with phosphorylated β-catenin (22, 26), is essential for the ubiquitination of β-catenin (33). Treatment with Bt2cAMP increased the β-catenin level in the cells expressing FLAG-Fbw1, and the amounts of β-catenin incorporated in the FLAG-Fbw1 immunoprecipitates from the cells treated with Bt2cAMP were more than from the cells without treatment (Fig. 6B). Bt2cAMP did not inhibit the formation of a complex between FLAG-Fbw1 and β-catenin, which was phosphorylated at Ser33, Ser37, and Thr41 (Fig. 6B). It has been shown that overexpression of Fbw1 induces the degradation of β-catenin (22). However, expression of Fbw1 in HEK-293 cells did not affect Bt2cAMP-induced nuclear localization of β-catenin (Fig. 6C). Furthermore, Fbw1 did not inhibit Bt2cAMP- but Wnt-3a-induced activation of Tcf-4 (Fig. 6D). These results suggest that PKA does not affect the formation of a complex between Fbw1 and the phosphorylated form and that Fbw1 does not stimulate the degradation of β-catenin when PKA is activated.
To examine how PKA inhibits the ubiquitination of β-catenin, we developed an in vitro reconstitution assay using purified proteins. The SCF complex (His6-T7-Skp1, HA-Cul1, GST-Myc-Fbw1, and Myc-Rbx1) was purified from Sf9 cells using TALON metal affinity resin (Fig. 6E). The ubiquitination of β-catenin was evident under the complete assay conditions (Fig. 6F, lane 1). In the absence of ubiquitin, β-catenin was phosphorylated at Ser33, Ser37, Thr41, and Ser45 but not ubiquitinated, and in the absence of ATP, neither phosphorylation nor ubiquitination of β-catenin was observed (Fig. 6F, lanes 2 and 3). Pretreatment of β-catenin with PKAc inhibited the ubiquitination of β-catenin, and H-89 prevented the inhibition of the ubiquitination of β-catenin by PKAc (Fig. 6F, lanes 4 to 6). No protein of the SCF complex was phosphorylated by PKAc (data not shown), and pretreatment of the SCF complex with PKA did not inhibit the ubiquitination of β-catenin (Fig. 6G). Phosphorylation of Axin by PKAc did not affect the ubiquitination of β-catenin either (data not shown). These results suggest that direct phosphorylation of β-catenin by PKA inhibits the ubiquitination of β-catenin.
Involvement of phosphorylation of Ser675 of β-catenin in PKA-dependent stabilization of β-catenin.
There are several possible sites (R/K-R/K-X-S/T) for phosphorylation by PKA in β-catenin. Among them, we found that Ser675 is at least one of the sites of phosphorylation by PKA, because substitution of Ser675 with Ala reduced the phosphorylation of β-catenin by PKAc in vitro but substitution of Thr510 with Ala did not affect the phosphorylation level (Fig. 7A, lanes 1 to 3). We then generated the antibody that specifically recognizes phosphorylated β-catenin at Ser675 (anti-pS675 Ab). Wild-type β-catenin but not β-cateninS675A was indeed recognized by the anti-pS675 antibody when each was phosphorylated by PKAc in vitro (Fig. 7A, lanes 4 to 7). We examined whether PKA phosphorylates β-catenin at Ser675 in intact cells. When L cells were treated with Bt2cAMP or PGE1, the anti-pS675 antibody recognized endogenous β-catenin, which corresponds to the phosphorylated β-catenin at Ser675 (Fig. 7A, lanes 8 to 11). HA-β-catenin but not HA-β-cateninS675A was detected by this antibody when HEK-293T cells expressing β-catenin exogenously were treated with Bt2cAMP (Fig. 7A, lanes 12 to 15). These results indicate that PKA can phosphorylate β-catenin at Ser675 in intact cells.
FIG. 7.
Identification of phosphorylation site of β-catenin that impairs ubiquitination. (A) Phosphorylation site of β-catenin. Two possible phosphorylation sites of β-catenin by PKA are indicated. Wild-type His6-β-catenin, His6-β-cateninS675A, and His6-β-cateninT510A (2 μg) were phosphorylated by PKAc, and the samples were subjected to autoradiography (lanes 1 to 3). Wild-type His6-β-catenin and His6-β-cateninS675A were incubated with or without PKAc. The samples (10 ng) were probed with the anti-β-catenin and anti-pSer675 antibodies (lanes 4 to 7). L cells were treated with Bt2cAMP, PGE1, or Wnt-3a. The lysates were immunoprecipitated with the anti-β-catenin antibody, and the equivalent amounts of β-catenin were probed with the anti-pSer675 antibody (lanes 8 to 11). After HEK-293T cells expressing HA-β-catenin (lanes 12 and 13) or HA-β-cateninS675A (lanes 14 and 15) were treated with Bt2cAMP, the lysates were immunoprecipitated with the anti-HA antibody and the equivalent amounts of β-catenin were probed with the indicated antibodies. (B) Pulse-chase analysis of β-catenin. COS cells expressing wild-type HA-β-catenin (circles), HA-β-cateninS675A (squares), or HA-β-cateninT510A (triangles) were subjected to pulse-chase analyses in the presence (filled symbols) or absence (open symbols) of Bt2cAMP and IBMX. β-Catenin and its mutants were immunoprecipitated with the anti-ΗΑ antibody, and the incorporation of 35S into β-catenin was scanned using the Molecular Dynamics Storm imaging system. Signals were quantified using ImageQuant software (Molecular Dynamics) and expressed as the percentage of the value of time zero. The results shown are means ± standard errors of five independent experiments. (C) Effects of PKA on ubiquitination of β-cateninS675A. The assay conditions were the same as those for Fig. 6F except that β-cateninS675A was used. (D) Effects of PKA on the Tcf-4 activity through β-cateninS675A. pCGN/β-catenin (0.5 μg), pCGN/β-cateninT510A (0.5 μg), pCGN/β-cateninS675A (0.5 μg), or pCGN/β-cateninSA (0.5 μg) was transfected into L cells with Axin2-Luc (0.5 μg) and pEF-BOS-HA/hTcf-4E (0.1 μg) or pEF-BOS-HA/hTcf-4E (Δ1-53) (0.1 μg) in the presence or absence of pCGN/PKAc (0.1 μg). After 36 h, the luciferase activity was measured and expressed as the fold increase compared with the level observed in the cells transfected with Axin2-Luc and pEF-BOS-HA/hTcf-4E. The results shown are means ± standard errors from four independent experiments. WT, wild type.
To investigate whether this serine residue is involved in PKA-dependent stabilization of β-catenin, pulse-chase analyses in COS cells expressing wild-type HA-β-catenin or its mutants were performed. Equivalent amounts of HA-β-catenin was immunoprecipitated with the anti-HA antibody from the lysates of COS cells expressing HA-β-catenin or its mutants (data not shown). Wild-type HA-β-catenin decreased with a half-life of 90 min, and treatment of COS cells with Bt2cAMP suppressed degradation of wild-type HA-β-catenin (Fig. 7B). Although HA-β-cateninS675A exhibited a degradation rate similar to that of wild-type HA-β-catenin, treatment of COS cells with Bt2cAMP did not affect the degradation of HA-β-cateninS675A. In contrast, the degradation of HA-β-cateninT510A was suppressed by Bt2cAMP to the same extent as that of wild-type HA-β-catenin (Fig. 7B). Consistent with these results, pretreatment of β-cateninS675A with PKAc did not inhibit the ubiquitination of β-catenin in the in vitro reconstitution assay (Fig. 7C).
To examine whether these β-catenin mutants activate Tcf-4 in a PKA-dependent manner, Axin2-Luc, PKAc, and β-catenin were expressed in L cells. Coexpression of PKAc enhanced the wild-type β-catenin- or β-cateninT510A-dependent activation of Tcf-4 towards the Axin2 promoter (Fig. 7D). These effects of PKAc were suppressed by H-89 (data not shown). However, PKAc only slightly enhanced the β-cateninS675A-dependent activation of Tcf-4 (Fig. 7D). β-CateninSA, another β-catenin mutant, in which Ser33, Ser37, Thr41, and Ser45 are mutated to Ala, is not degraded by proteasome. β-CateninSA activated Tcf-4 more strongly than did wild-type β-catenin, and coexpression of PKAc did not affect β-cateninSA-dependent Tcf-4 activity (Fig. 7D). As controls, wild-type β-catenin or its mutants did not activate Tcf-4-(Δ1-53) in the presence or absence of PKAc. Taken together, these results suggest that the phosphorylation of β-catenin by PKA inhibits its ubiquitination, resulting in the accumulation of β-catenin and the activation of Tcf-4.
DISCUSSION
In this study we found that the activation of PKA leads to an increase in the protein level of β-catenin and that PKA can regulate gene expression through β-catenin and Tcf-4. Since Bt2cAMP did not affect the β-catenin mRNA level but inhibited the degradation of the β-catenin protein, it was thought that PKA regulates the posttranslational stabilization of β-catenin. Phosphorylation of GSK-3β, β-catenin, and Axin by PKA did not affect the mutual binding, and PKA did not affect the stability of Axin. Therefore, we investigated the effects of PKA on the phosphorylation and ubiquitination of β-catenin.
Mechanism of stabilization of β-catenin by PKA.
PKA inhibited GSK-3 in the Axin complex, but to a lesser degree than GSK-3 free from Axin. These results are similar to the observations that insulin does not inhibit the GSK-3β activity in the conductin (Axil) complex (6). GSK-3β complexed with Axin may be resistant to inhibition by other protein kinases. PKA also phosphorylated Ser45 of β-catenin and enhanced the phosphorylation at Ser33, Ser37, and Thr41 of β-catenin induced by GSK-3β in intact cells and in vitro. The result in intact cells may reflect a decrease in relative phosphorylation of β-catenin. The stabilization of β-catenin due to the inhibition of GSK-3 by PKA may be greater than the degradation of β-catenin due to the phosphorylation of β-catenin of Ser45 by PKA. This possibility is consistent with the previous observations that PGE2, which also activates PKA, activates Tcf probably through the phosphorylation and inhibition of GSK-3 (8). Therefore, one possible mechanism for PKA to induce the accumulation of β-catenin might be due to the PKA-dependent inhibition of GSK-3β.
However, we have raised another possible mechanism, i.e., that PKA phosphorylates serine or threonine residues other than Ser45 in β-catenin, thereby stabilizing β-catenin. Ser675 of β-catenin was found to be a site of phosphorylation by PKA in intact cells. Since the inhibition of the ubiquitination and degradation by PKA was not observed in β-cateninS675A, the phosphorylation of this serine residue by PKA is important for the accumulation of β-catenin by PKA. This conclusion is also supported by the finding that PKA does not enhance the β-cateninS675A-dependent activation of Tcf-4. Furthermore, PKA did not affect the binding of Fbw1 to phosphorylated β-catenin. We conclude that PKA-dependent stabilization of β-catenin is at least partly due to the inhibition of the ubiquitination of β-catenin through the phosphorylation of Ser675 of β-catenin by PKA. We do not know the molecular mechanism by which PKA inhibits the ubiquitination of β-catenin at present. One possibility is that ubiquitination of lysine residues of β-catenin may be impaired by changes of its three-dimensional structure by PKA-dependent phosphorylation. Whole structures of the Axin complex containing GSK-3, β-catenin, and APC remain to be clarified.
Cross talk between Wnt and PKA pathways.
This study demonstrated that PKA inhibits the ubiquitination of β-catenin, resulting in the accumulation of β-catenin. Wnt inhibits the GSK-3β-dependent phosphorylation of β-catenin (26, 43) or induces the downregulation of Axin (39, 41), thereby leading to the accumulation of β-catenin. It has been reported that tyrosine phosphorylation of β-catenin increases its cytoplasmic protein level, although the mechanism is largely unknown (34). Therefore, the accumulation of β-catenin by PKA is the new mechanism to stabilize β-catenin. Our results are not consistent with the previous observations that β-catenin is degraded as a result of the phosphorylation by PKA in the presenilin complex (15). Since β-catenin is stabilized in presenilin-deficient cells (15), it seems that presenilin is necessary for the degradation of β-catenin. Therefore, phosphorylation of β-catenin by PKA in the presenilin complex may lead to ubiquitination, and the action of PKA on β-catenin complexed with presenilin may be different from that of PKA on β-catenin complexed with Axin.
The activation of PKA suppresses the proliferation of most types of cells, whereas it stimulates the cellular growth of endocrine cells. Mutations of the α subunit of oligomeric GTP-binding proteins that activate adenylate cyclase were found in human pituitary tumors producing growth hormone (24) and in thyroid adenoma (29). Therefore, it is intriguing to speculate that constitutive activation of PKA stabilizes β-catenin and activates Tcf, resulting in abnormal cell growth of these tumor cells.
It has been shown that the PKA pathway is involved in the Wnt-regulated development of skeletal muscles (5). Wnt-1, -3a, and -7a activate PKA through trimeric GTP-binding proteins, resulting in CREB (cAMP-responsive element-binding protein)-dependent transcriptional activation in C57MG and 10T1/2 cells. However, Wnt-3a did not produce cAMP in L and HEK-293 cells (data not shown). These results are consistent with the results that H-89 did not inhibit Wnt-3a-dependent accumulation of β-catenin and activation of Tcf-4 in these cells. Therefore, whether Wnt activates PKA or not may be dependent on cell type. It remains to be clarified that the PKA pathway functions as a positive feedback loop of the Wnt signal pathway and that Wnt regulates both the Tcf- and CREB-dependent transcription.
Acknowledgments
We are grateful to D. M. Virshup, M. Nakao, R. Lefkovitz, F. Costantini, and H. Clevers for donating plasmids.
This work was supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture, Japan (2002, 2003, and 2004), and by grants from the Yamanouchi Foundation for Research on Metabolic Disorders (2003).
REFERENCES
- 1.Bengoechea-Alonso, M. T., B. Pelacho, J. A. Osés-Prieto, E. Santiago, N. López-Moratalla, and M. J. López-Zabalza. 2003. Regulation of NF-κB activation by protein phosphatase 2B and NO, via protein kinase A activity, in human monocytes. Nitric Oxide 8:65-74. [DOI] [PubMed] [Google Scholar]
- 2.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 4.Brunton, L. L., R. A. Wiklund, P. M. Van Arsdale, and A. G. Gilman. 1976. Binding of [3H]prostaglandin E1 to putative receptors linked to adenylate cyclase of cultured cell clones. J. Biol. Chem. 251:3037-3044. [PubMed] [Google Scholar]
- 5.Chen, A. E., D. D. Ginty, and C. M. Fan. 2005. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature 433:317-322. [DOI] [PubMed] [Google Scholar]
- 6.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 7.Fang, X., S. X. Yu, Y. Lu, R. C. J. Bast, J. R. Woodgett, and G. B. Mills. 2000. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. USA 97:11960-11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujino, H., K. A. West, and J. W. Regan. 2002. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J. Biol. Chem. 277:2614-2619. [DOI] [PubMed] [Google Scholar]
- 9.He, X., M. Semenov, K. Tamai, and X. Zeng. 2004. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131:1663-1677. [DOI] [PubMed] [Google Scholar]
- 10.Hino, S.-I., T. Michiue, M. Asashima, and A. Kikuchi. 2003. Casein kinase Iɛ enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of β-catenin. J. Biol. Chem. 278:14066-14073. [DOI] [PubMed] [Google Scholar]
- 11.Hinoi, T., H. Yamamoto, M. Kishida, S. Takada, S. Kishida, and A. Kikuchi. 2000. Complex formation of adenomatous polyposis coli gene product and Axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J. Biol. Chem. 275:34399-34406. [DOI] [PubMed] [Google Scholar]
- 12.Ihara, M., H. Yamamoto, and A. Kikuchi. 2005. SUMO-1 modification of PIASy, an E3 ligase, is necessary for PIASy-dependent activation of Tcf-4. Mol. Cell. Biol. 25:3506-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamura, T., T. Hara, S. Kotoshiba, M. Yada, N. Ishida, H. Imaki, S. Hatakeyama, K. Nakayama, and K.-I. Nakayama. 2003. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl. Acad. Sci. USA 100:10231-10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, D. E., S. Soriano, X. Xia, C. G. Eberhart, B. De Strooper, H. Zheng, and E. H. Koo. 2002. Presenilin couples the paired phosphorylation of β-catenin independent of axin: implications for β-catenin activation in tumorigenesis. Cell 110:751-762. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi, A. 1999. Roles of Axin in the Wnt signalling pathway. Cell. Signal. 11:777-788. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi, A. 2003. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 94:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishida, M., S.-I. Hino, T. Michiue, H. Yamamoto, S. Kishida, A. Fukui, M. Asashima, and A. Kikuchi. 2001. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iɛ. J. Biol. Chem. 276:33147-33155. [DOI] [PubMed] [Google Scholar]
- 19.Kishida, M., S. Koyama, S. Kishida, K. Matsubara, S. Nakashima, K. Higano, R. Takada, S. Takada, and A. Kikuchi. 1999. Axin prevents Wnt-3a-induced accumulation of β-catenin. Oncogene 18:979-985. [DOI] [PubMed] [Google Scholar]
- 20.Kishida, S., H. Yamamoto, S.-I. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19:4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishida, S., H. Yamamoto, S. Ikeda, M. Kishida, I. Sakamoto, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J. Biol. Chem. 273:10823-10826. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, K.-I. Nakayama, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 24.Landis, C. A., S. B. Masters, A. Spada, A. M. Pace, H. R. Bourne, and L. Vallar. 1989. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340:692-696. [DOI] [PubMed] [Google Scholar]
- 25.Li, G., and R. Iyengar. 2002. Calpain as an effector of the Gq signaling pathway for inhibition of Wnt/β-catenin-regulated cell proliferation. Proc. Natl. Acad. Sci. USA 99:13254-13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, C., Y. Li, M. Semenov, C. Han, G.-H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 27.Liu, J., J. Stevens, C. A. Rote, H. J. Yost, Y. Hu, K. L. Neufeld, R. L. White, and N. Matsunami. 2001. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell 7:927-936. [DOI] [PubMed] [Google Scholar]
- 28.Lustig, B., B. Jerchow, M. Sachs, S. Weiler, T. Pietsch, U. Karsten, M. van de Wetering, H. Clevers, P. M. Schlag, W. Birchmeier, and J. Behrens. 2002. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22:1184-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons, J., C. A. Landis, G. Harsh, L. Vallar, K. Grunewald, H. Feichtinger, Q. Y. Duh, O. H. Clark, E. Kawasaki, and H. R. Bourne. 1990. Two G protein oncogenes in human endocrine tumors. Science 249:655-659. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzawa, S.-I., and J. C. Reed. 2001. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell 7:915-926. [DOI] [PubMed] [Google Scholar]
- 31.Montminy, M. R., K. A. Sevarino, J. A. Wagner, G. Mandel, and R. H. Goodman. 1986. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Natl. Acad. Sci. USA 83:6682-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murai, H., M. Okazaki, and A. Kikuchi. 1996. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 392:153-160. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama, K., S. Hatakeyama, S. Maruyama, A. Kikuchi, K. Onoe, R. A. Good, and K.-I. Nakayama. 2003. Impaired degradation of inhibitory subunit of NF-κB (IκB) and β-catenin as a result of targeted disruption of the β-TrCP1 gene. Proc. Natl. Acad. Sci. USA 100:8752-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson, W. J., and R. Nusse. 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 36.Rubinfeld, B., I. Albert, E. Porfiri, C. Fiol, S. Munemitsu, and P. Polakis. 1996. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272:1023-1026. [DOI] [PubMed] [Google Scholar]
- 37.Seidensticker, M. J., and J. Behrens. 2000. Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta 1495:168-182. [DOI] [PubMed] [Google Scholar]
- 38.Tanji, C., H. Yamamoto, N. Yorioka, N. Kohno, K. Kikuchi, and A. Kikuchi. 2002. A-kinase anchoring protein AKAP220 binds to glycogen synthase kinase-3β (GSK-3β) and mediates protein kinase A-dependent inhibition of GSK-3β. J. Biol. Chem. 277:36955-36961. [DOI] [PubMed] [Google Scholar]
- 39.Tolwinski, N. S., M. Wehrli, A. Rives, N. Erdeniz, S. DiNardo, and E. Wieschaus. 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3β activity. Dev. Cell 4:407-418. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, H., M. Ihara, Y. Matsuura, and A. Kikuchi. 2003. Sumoylation is involved in β-catenin-dependent activation of Tcf-4. EMBO J. 22:2047-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto, H., S. Kishida, M. Kishida, S. Ikeda, S. Takada, and A. Kikuchi. 1999. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J. Biol. Chem. 274:10681-10684. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto, H., S. Kishida, T. Uochi, S. Ikeda, S. Koyama, M. Asashima, and A. Kikuchi. 1998. Axil, a member of the Axin family, interacts with both glycogen synthase kinase-3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 18:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagawa, S.-I., Y. Matsuda, J.-S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]