Abstract
Exposure of primary cells to mitogenic stimuli or oncogenes often causes them to undergo premature senescence. This is most likely a protective function that prevents uncontrolled proliferation. Pak4 is a target for the Rho GTPase Cdc42. Pak4 is overexpressed in human tumor cell lines, and it is the only member of the Pak family that is highly transforming in immortalized fibroblasts. Here we show that in primary fibroblasts, activated Pak4 inhibits cell proliferation and promotes premature senescence. Furthermore, Pak4 expression levels are upregulated in response to stimuli that promote senescence. Pak4-induced arrest appears to be mediated by a pathway that requires the ERK mitogen-activated protein kinase, as well as the cell cycle inhibitors p16INK4 and p19ARF. These new results describing a role for Pak4 in senescence are important for understanding why this protein is associated with cancer and how it promotes transformation in immortalized cells.
Normal cells live only for a limited number of generations, after which they typically undergo replicative senescence. Replicative senescence is closely associated with the process of telomere shortening (17). Senescent cells remain metabolically active but have no possibility of undergoing further divisions. Senescent fibroblasts typically display a large, flat shape, have high acidic β-galactosidase (SA-β-Gal) activity, and exhibit specific changes in gene expression (7, 13, 39). While replicative senescence is a normal process that occurs as cells reach the end of their life-span, another type of senescence is premature, nonreplicative senescence (7). Premature senescence can be triggered by various types of DNA damage, chromatin remodeling, and strong mitogenic stimuli. In primary cells, certain oncogenes have also been shown to promote premature senescence, sometimes after an initial boost of proliferation (10, 22, 38, 47). Under these conditions, premature senescence is probably a protective response that prevents uncontrolled proliferation triggered by oncogenes or proliferation of damaged cells.
The Ras oncogene is a small GTP binding protein that has been shown to trigger premature senescence in primary cells (22, 38), even though it is highly transforming in immortalized cells. Likewise, oncogenic Raf, a Ras target, can also promote premature senescence in primary cells (22, 47). Premature cellular senescence induced by oncogenes such as Ras is associated with increased levels of p16INK4a and p19ARF via a pathway that most likely involves Raf-MEK-ERK signaling (5, 22). p16INK4a is a cyclin-dependent kinase inhibitor that inhibits the activities of cyclin-dependent kinases 4/6. This in turn leads to inhibition of retinoblastoma phosphorylation, thereby inhibiting cell cycle progression. In contrast, p19ARF stabilizes the tumor-suppressor protein p53 by interfering with its negative regulator MDM2. p53 in turn induces the expression of the cyclin-dependent kinase inhibitor p21CIP1, which can inhibit cyclin E- and A-dependent kinase complexes and thus promote cell cycle arrest. p19ARF clearly has an important role in senescence, since cells that are deficient in p19ARF no longer undergo senescence in response to oncogenic Ras (29, 40).
In addition to oncogenic Ras, other small GTP binding proteins also have important roles in oncogenesis. Of particular interest is the family of Rho GTPases, consisting of Cdc42, Rac1, RhoA, and other family members. The Rho proteins were originally identified as proteins that regulate cell morphology and the actin cytoskeleton (15). However, they also regulate gene expression, cell proliferation, and cell survival (36). All of these cellular functions are thought to play important roles in tumorigenesis. Rho proteins are required in Ras-induced malignant transformation, and activated mutants of the GTPases, which are weakly transforming on their own, can significantly enhance the focus-forming potential of membrane-targeted Raf (20, 31-34). Moreover many guanine nucleotide exchange factors, which promote Rho protein activation by exchanging GDP for GTP, have been isolated as activated forms in screens for transforming genes (8). These proteins, members of the Dbl family of exchange factors, are potent oncogenes (8), and activation of Rho GTPases is thought to be responsible for their transforming ability.
In order to understand how the Rho GTPases regulate growth and transformation, it is important to determine which target proteins mediate these processes. In their GTP-bound, activated forms, the Rho GTPases bind and activate a number of different target proteins (4). Among these are the Pak family of serine/threonine kinases, which bind to activated Cdc42 and Rac. The Pak family of kinases can be divided into two subgroups, based on their amino acid sequences and functions. The first group, group A, consists of mammalian Pak1, -2, and -3, which are all quite similar in sequence. The second group is group B, which consists of Pak4, -5, and -6 (19).
Several studies have indicated that activated mutants of the group A Paks do not transform cells (41-43), and a direct role for the group A Paks in transformation by the Rho family GTPases has not been demonstrated. In contrast to the group A Paks, however, Pak4 is highly transforming. Like activated Cdc42 (23, 24, 32), activated Pak4 promotes anchorage-independent growth in fibroblasts, an important hallmark of oncogenic transformation (35). In fact, activated Pak4 is as efficient as oncogenic Ras in promoting anchorage-independent growth. Dominant-negative Pak4 also blocks transformation by oncogenic Dbl (35) or Ras (6), indicating that it is an important player in the pathway leading from Rho GTPases to transformation. Not only is Pak4 associated with transformation in cell culture systems, but recent studies indicate that Pak4 overexpression is associated with human cancers. Although Pak4 is poorly expressed in most normal adult tissues, it was found to be highly overexpressed in a panel of tumor cell lines (6). Taken together, these results suggest an important role for Pak4 in transformation and indicate that overexpression of Pak4 may be linked to human cancers.
Since Pak4 has been shown to be highly transforming in immortalized cells, we have studied the role for Pak4 in primary fibroblasts. Primary fibroblasts are an interesting model system because they do not have genetic mutations associated with immortalization and are thus not predisposed to transformation. Interestingly, in primary cells Pak4 did not promote transformation but instead led to premature senescence. Similarly, oncogenic Dbl, which lies upstream to Pak4, also promoted premature senescence. Furthermore, Pak4 levels were upregulated by oncogenes known to promote premature senescence. Pak4-induced premature senescence appeared to be mediated by the ERK pathway and required the presence of p19ARF and p16INK4a. Our results indicate that similar to strong oncogenes such as Ras and Raf, Pak4 activates a signaling pathway leading to premature senescence in primary fibroblasts. This is the first study to show a role for a Rho GTPase effector protein in the signaling pathway leading to premature senescence, and it points to a new function for Pak family kinases.
MATERIALS AND METHODS
Cell culture, gene transfer, and vectors.
All cell lines were cultured in Dulbecco's modified Eagle's medium supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin, and 4 mM glutamine and either 10% bovine calf serum (NIH 3T3 cells) or 10% fetal bovine serum (primary fibroblasts and 293 cells). Transient transfections of NIH 3T3 cells were carried out using the LipofectAMINE method (Life Technologies, Inc.) according to the manufacturer's protocol. Cells were seeded at a density of 3.7 × 105/3.5-cm diameter dish and were starved 24 h after transfection in 0.2% serum. 293 cells were transfected using a standard calcium phosphate precipitation method.
Primary mouse embryo fibroblasts from wild-type (Swiss Webster or B6/129 mice) and INK4a/ARF−/− day 13.5 embryos were prepared as described previously (30). Ink4/ARF null mice are described in reference 37. Pak4−/− and Pak4+/+ embryonic fibroblasts were prepared at embryonic day 10.5 from littermate embryos obtained from a cross of PAK4 heterozygous (+/−) mice, as previously described (35a). Activated Pak4 (S445N) (35), (referred to here as PAK4*), oncogenic Ras (RasV12), oncogenic Dbl, and activated PAK1T423E (referred to here as PAK1*) were expressed using the pLPC retroviral vector. Retroviral gene transfer was performed as described previously (30) using high-titer retroviral stocks generated by transient transfection of the Phoenix ecotropic packaging cell line (G. Nolan, Stanford University, CA). Infected cell populations were selected by culture in puromycin (2.5 μg/ml, 3 to 4 days) to eliminate uninfected cells. Day 5 postinfection was designated as day 0. In all experiments, cells infected with empty pLPC vector (EV) were used as a control.
Cell proliferation and SA-β-Gal assays.
For growth curves, infected populations of cells were plated at a density of 1.5 × 104 (except PAK4+/+ and PAK4−/− cells, which were plated at 3 × 104 cells per well in 12-well plates) cells per well in 12-well plates following drug selection. Cells were harvested by trypsinization at intervals of 48 h, stained with 0.1% trypan blue, and counted by use of a hemocytometer. Each growth curve was performed at least twice, and each point was done in triplicate and the results averaged. For experiments using PD98059 (Calbiochem), treatment was initiated 2 days postinfection, and fresh medium containing 50 μM PD98059 was added daily. For BrdU incorporation, subconfluent cultures (6 × 104 cell per 35-mm-diameter plate) were plated on coverslips and labeled with a labeling solution (30 μM BrdU, 30 μM 2-deoxycytidine, and 10 μM 5-fluorodeoxyuridine) for 3 h prior to harvesting. Cells were then fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and treated with DNase I (0.5 mg/ml; Sigma) at 30°C for 20 min. Coverslips were incubated with a monoclonal antibody against BrdU (5 μg/ml; Boehringer-Mannheim) for 60 min followed by incubation with a rhodamine-conjugated antimouse secondary antibody (Pierce) in a solution containing 1 μg/ml 4′,6′diamidino-2-phenylindole. For senescence-associated β-galactosidase detection, cells were plated at a density of 4 × 104 in 3.5-cm plates and treated as described previously (30, 38).
Protein expression and activity.
For Western blots and protein kinase assays, cells were trypsinized, washed once in phosphate-buffered saline, and lysed in M2 buffer [20 mM Tris-Hcl [pH 7.6], 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerophosphate, 1 mM Na3(VO)4, 1 μg/ml leupeptin] for 20 min and then cleared by centrifugation. Western blots were performed using 20 μg of whole-cell lysates according to standard procedures using ECL detection (Amersham). The following antibodies were used: anti-mouse p53 (1:2,500 CM5; Novocastra), anti-p19ARF (1:500; Novus), anti-p21 (0.5 mg/ml SX118; Pharmingen), anti-p16 (1:500 M156; Santa Cruz), and antiactin (1:3,000 AC-40; Sigma). PAK4 expression levels were assessed either with a monoclonal anti-Myc antibody or with a monoclonal anti-PAK4 antibody. Protein expression levels were obtained using the ImageJ quantification program.
For Pak4, Pak1, and ERK kinase assays, approximately 100 μg of cell extract was incubated with the appropriate antibody for 2 to 3 h at 4°C. Subsequently, 25 μl of protein A-Sepharose beads (50%) were added and the mixture incubated for an additional 2 h at 4°C. Either a polyclonal anti-ERK2 antibody (1 μg/ml; Santa Cruz) or an anti-Myc antibody (1 μg/ml 9E10; Santa Cruz) was used. Immune complexes were then washed twice in M2 buffer and twice in kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2) and incubated at 30°C in 30 μl of kinase buffer containing 20 mM glycerophosphate, 20 mM p-nitrophenyl phosphate, 1 mM dithiothreitol, 50 μM Na3V04, 20 μM ATP, and 5 μCi of [γ-32P]ATP. Five micrograms of myelin basic protein (MBP) (Sigma) was used as a substrate in ERK kinase assays, and autophosphorylation of Pak4 was used as a marker of activity in Pak4 kinase assays. Reactions were stopped after 20 min by denaturation in sodium dodecyl sulfate (SDS) loading buffer. Proteins were resolved on SDS-polyacrylamide gels, and substrate phosphorylation was visualized by autoradiography.
For in vitro Raf phosphorylation assays, 293 cells were transfected with either Myc-tagged PAK4 or M2-tagged Raf1. The proteins were immunopurified from cell extracts using anti-Myc (1 μg/ml 9E10; Santa Cruz) or anti-M2 (1 μg/ml; Sigma) antibodies. The two immunoprecipitates were then mixed together and incubated at 30°C for 1 h in kinase buffer in the presence of 500 μM ATP and no radioactive isotope. Proteins were resolved on SDS-polyacrylamide gels, and substrate phosphorylation was visualized by Western blotting. The antibodies used were anti-Raf (1:200 C12; Santa Cruz) and anti-phospo-Raf1(Ser338) (1:1,000; Upstate Biotechnology). For Raf kinase assays, 200 μg of cell extract was incubated with anti-M2 antibody to pull down transfected Raf. Kinase assays were performed using the Raf-1 immunoprecipitation kinase cascade assay kit (Upstate Biotechnology) according to the manufacturer's instructions.
Transformation assays.
For soft agar assays, cells were resuspended in 0.3% Noble agar (Becton Dickinson) in Dulbecco's modified Eagle's medium supplemented with 10% serum, 100 μM sodium pyruvate, and 10 μM nonessential amino acids at a density of 1 × 104 cells per 35-mm well. Cells were then plated onto solidified 0.5% Noble agar-containing medium. Fresh medium was added to the cells weekly, and photomicrographs of colonies were taken 2 weeks later. For experiments using U0126 and SB203580 (both from Calbiochem), cells were plated in medium containing 20 μM U0126 or 10 μM SB203580, and 500 μl of fresh medium containing the appropriate amount of drug was added every 3 days.
RESULTS
Pak4 causes growth arrest and senescence.
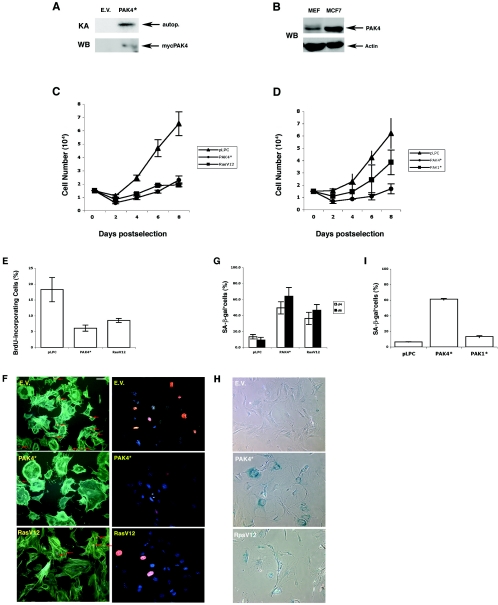
We have previously found that activated Pak4 transforms immortalized fibroblasts (35). To see how Pak4 affects the growth of normal primary fibroblasts, we infected primary mouse embryo fibroblasts (MEFs) with retroviruses encoding an activated form of Pak4 along with a puromycin resistance gene. Vectors containing puromycin alone or puromycin plus oncogenic Ras were used as negative and positive controls, respectively. Pooled populations of infected cells were selected with puromycin for 3 to 4 days, and the fifth day postinfection was designated day 0. As shown in Fig. 1A, at day 2 we observed an increase in Pak4 protein levels and kinase activity in the Pak4-infected cells. Importantly, although Pak4 was overexpressed, its expression was not higher than the level seen in cancer cell lines, such as the MCF7 breast cancer cell line, as shown in Fig. 1B.
FIG. 1.
Pak4 induces senescence in primary MEFs. A. Expression and activity of ectopic Pak4 in infected cells at day 2 postselection. Primary MEFs were infected with retroviruses expressing activated PAK4 (PAK4*), empty vector (E.V.), or H-RasV12. At day 2 postselection, Myc-PAK4 protein levels were assessed by Western blotting with an antibody directed against the Myc epitope. PAK activity was verified by immunocomplex kinase assay using autophosphorylation as a marker for kinase activity. B. Pak4 levels in infected MEFs are lower than the levels in cancer cell lines. Cell extracts were prepared from MCF7 breast cancer cells and from primary fibroblasts (MEFs) infected with activated Myc-tagged Pak4. Equal amounts of protein from each sample were subject to Western blot analysis using a monoclonal antibody directed against PAK4 (upper panel) or actin (bottom panel). C. Expression of Pak4 inhibits growth of primary MEFs. Representative growth curves of primary MEFs infected with empty pLPC vector (triangles), activated PAK4* (circles), or RasV12 (squares) are shown. D. Expression of Pak1 does not inhibit growth of primary MEFs. Representative growth curves of primary MEFs infected with empty pLPC vector (triangles), activated Pak4 (PAK4*; closed circles), or activated Pak1 (PAK1*; closed squares) are shown. E and F. Decrease in BrdU incorporation in cells expressing Pak4. Percentage of BrdU-incorporating cells at day 6 postselection in cell populations infected with empty vector, activated PAK4 (PAK4*), or RasV12 is indicated. Incorporated BrdU was detected by immunofluorescence using an anti-BrdU antibody. About 200 cells from each population were scored, and the average and standard deviation for at least three experiments are shown. Typical fields of cells are shown in panel F. Left: BrdU-incorporating nuclei are stained orange with rhodamine-conjugated secondary antibody and indicated by an arrow. The cytoskeleton is stained green with fluorescein isothiocyanate-labeled phalloidin. Right: different fields of the same preparation of cells treated with BrdU and stained for both BrdU (orange) and 4′,6′diamidino-2-phenylindole (blue). Bar, 20 μm. G and H. Expression of Pak4 leads to an increase in SA-β-Gal expression. Percentages of cells positive for SA-β-Gal (at pH 6.0) at day 4 (open bars) and at day 6 (closed bars) postselection are indicated. Cells were scored as in panel E. Typical fields of cells are shown in panel H. SA-β-Gal-positive cells stain blue. I. SA-β-Gal levels are not elevated in response to Pak1 in MEFs. Percentages of cells positive for SA-β-Gal (at pH 6.0) at day 6 postselection are shown. Cells were scored as in panel E.
The proliferative properties of the cell populations were monitored by growth curves and by BrdU labeling. Cells transduced with activated Pak4, empty vector, or RasV12 were plated at low density, and cell numbers were determined every 2 days starting at day 0 (Fig. 1C). The control population grew steadily until the cells became confluent, whereas Pak4-transduced cells stopped proliferating well before reaching confluence (Fig. 1C). For comparison, we also analyzed cells infected with activated Pak1. Unlike Pak4, Pak1 slowed cell growth somewhat but did not cause primary cells to stop growing (Fig. 1D), although they grew slightly more slowly than parental cells. Pak4-infected cells were therefore analyzed in more detail. BrdU labeling revealed that while the percentage of BrdU-positive cells was high in control populations, only a small number of cells transduced with Pak4 incorporated BrdU by day 4 (data not shown) and virtually none by day 6 (Fig. 1E and F). Although they stopped growing, the population density of Pak4-infected cells did not decrease, and the cells remained attached to the plates for an extended period of time (data not shown). Interestingly, Pak4-expressing cells showed a large and flat shape. This phenotype was even more pronounced in the Pak4-expressing cells than in the Ras-infected cells. In addition, Pak4-expressing cells had a high concentration of actin around their periphery (Fig. 1F).
Both a flat enlarged cellular shape and subconfluent growth arrest in the presence of serum have been described as characteristics of senescence. Therefore, to determine whether Pak4-infected cells were undergoing senescence, we tested the cells for senescence-associated acidic β-galactosidase (SA-β-gal) activity, which has been associated with senescence in normal cells (13). We found that Pak4-infected cells stained positive for SA-β-Gal activity. This became apparent within 3 to 4 days and reached 60% of the population by day 6 (Fig. 1G and H). This was not observed in Pak1-infected cells (Fig. 1I). These results indicate that expression of Pak4, but not Pak1, leads to the onset of senescence in primary cells.
Pak4 is downstream to several oncogenes that induce senescence and plays a key role in the senescence pathway.
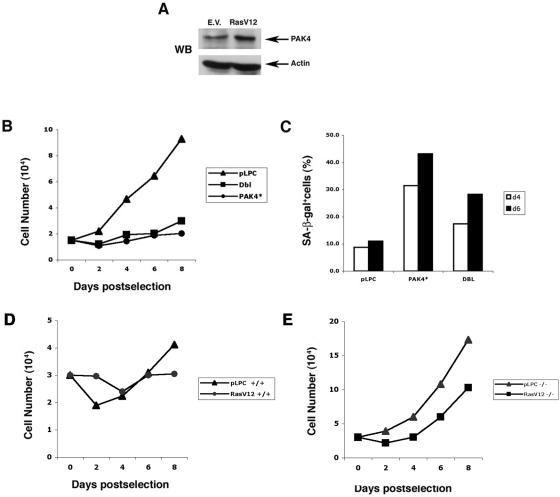
Several oncogenes were recently shown to promote premature senescence in primary fibroblasts, including oncogenic Ras and Raf (22, 38, 47). Interestingly, we found that expression of oncogenic Ras led to an increase in Pak4 protein levels in primary cells (Fig. 2A). Furthermore, oncogenic Dbl, which functions upstream to the Pak4 activator Cdc42 (16, 28), also promotes premature senescence (Fig. 2B and C). To determine whether Pak4 plays a necessary role in senescence pathways, we infected Pak4 wild-type and Pak4 null mouse primary fibroblasts with oncogenic Ras. These cells were isolated from very young (E10.5) embryos, which grew poorly compared with the MEFs used in the other studies, which had been isolated from E13.5 embryos, especially following retroviral infection. Interestingly, we found that Pak4 null cells infected with empty vector grew more rapidly and underwent less senescence than the wild-type cells. Furthermore, when infected with oncogenic Ras, the knockout cells did not undergo senescence, although they did grow somewhat more slowly than the empty vector-infected cells (Fig. 2D and E). Taken together, these results suggest an important role for Pak4 in cellular senescence pathways.
FIG. 2.
Pak4 protein levels increase in response to stimuli that induce senescence. A. PAK4 levels in RasV12-infected MEFs. Cell extracts were prepared from primary fibroblasts infected with recombinant retroviruses encoding either oncogenic Ras (RasV12) or empty vector (E.V.). Equal amounts of lysates from each sample were subjected to Western blot analysis using a monoclonal antibody directed against PAK4 (upper panel) or actin (bottom panel). B. Expression of oncogenic Dbl inhibits growth of primary MEFs. Representative growth curves of primary MEFs infected with empty pLPC vector, activated PAK4 (PAK4*), or oncogenic Dbl are shown. C. Expression of oncogenic Dbl leads to an increase in SA-β-Gal expression. Cells were infected with empty vector, activated Pak4, or oncogenic Dbl. Percentages of cells positive for SA-β-Gal (at pH 6.0) at day 4 (open bars) and at day 6 (closed bars) postselection are indicated. Cells were scored as in Fig. 1c. D and E. PAK4 role in premature senescence induced by oncogenic Ras. Representative growth curves of wild-type MEFs or PAK4 null MEFs infected with empty pLPC vector or oncogenic Ras are shown.
Expression of cell cycle regulatory proteins in response to Pak4 in primary cells.
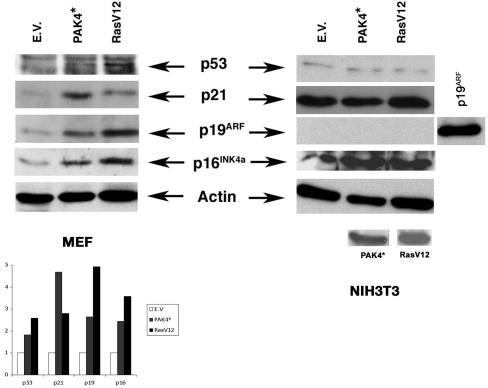
Our results indicate that Pak4 promotes premature senescence and that it is upregulated in response to several stimuli that promote senescence. To characterize further the nature of the cell cycle arrest caused by Pak4, we looked at the expression of several cell cycle regulatory proteins in Pak4-infected primary cells. The changes that distinguish cellular senescence from quiescence are thought to include up-regulation of the tumor suppressors p53 and p19ARF, along with the cell cycle inhibitors p16INK4a and p21CIP. Consistent with this, cells containing activated PAK4 displayed a moderate increase in p53 expression, along with a considerable increase in its transcriptional target p21CIP1 (Fig. 3). Furthermore, activated Pak4 led to a significant increase in p16INK4a and p19ARF levels (Fig. 3). These data, along with the accumulation of senescence-associated β-galactosidase, strongly suggest that the cell cycle arrest induced by activated Pak4 is indeed cellular senescence. In contrast to primary cells, in immortalized NIH 3T3 cells, overexpression of Pak4 did affect the levels of any of the cell cycle inhibitors or tumor suppressors that were tested (Fig. 3).
FIG. 3.
Pak4 stimulates the expression of cell cycle inhibitory proteins. Immunoblots of lysates of primary MEFs (left side) and NIH 3T3 cells (right side) infected or transfected, respectively, with empty vector (E.V.), activated PAK4 (PAK4*), or RasV12. Lysates were prepared 2 days following infection. Blots were probed with the indicated antibodies. Quantifications of the protein levels relative to the empty vector control are shown. The y axis shows the fold increase over empty vector. An antiactin antibody was used as a loading control. (NIH 3T3 cells transfected with a mouse p19ARF[p19ARF] vector were used as a positive control for p19, since no background band could be seen in these cells).
Pak4 induced senescence requires p16INK4 and p19ARF.
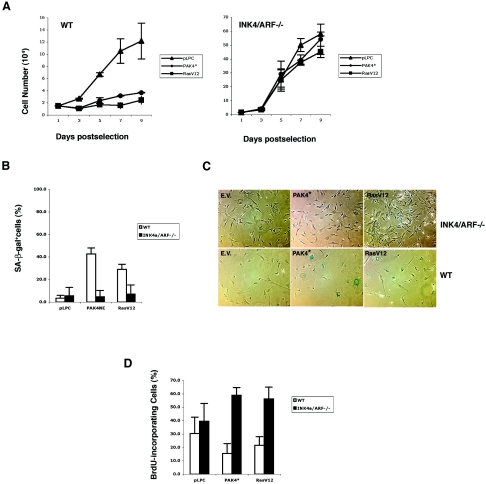
Since expression of Pak4 leads to increased levels of cell cycle regulatory proteins, we asked whether these proteins were also required for Pak4-induced senescence. To test this, we infected primary MEFs lacking p19ARF and p16INK4a with either empty vector, activated Pak4, or activated Ras. As expected, the control wild-type MEFs were readily arrested after infection with activated Pak4 or RasV12 (Fig. 4A). However, when Pak4 was expressed in primary p19ARF/p16INK4a null MEFs (37), the cells continued to grow (Fig. 4A and D) and did not senesce (Fig. 4B and C).
FIG. 4.
p16INK4a and p19ARF are required for Pak4-induced premature senescence. A. Pak4 does not inhibit growth in primary MEFs lacking p16INK4a and p19ARF. Representative growth curves are shown of wild-type MEFs or Ink4a/Arf null MEFs (37) transduced with empty vector (pLPC) or activated PAK4 (PAK4*)- or RasV12-expressing retroviruses. B and C. SA-β-Gal levels are not elevated in response to Pak4 in Ink4a/Arf null cells. Percentages of cells positive for SA-β-Gal (at pH 6.0) at day 6 postselection are shown. (White bars are wild-type cells; black bars are Ink4a/ARF null cells.) Typical fields of cells are shown in panel C. SA-β-Gal-positive cells stain blue. D. BrdU incorporation is not decreased in Ink4a/ARF null MEFs infected with Pak4. The percentages of BrdU-positive cells in wild-type or Ink4a/ARF null cells infected with empty vector, activated PAK4 (PAK4*), or RasV12 are indicated.
Pak4-induced senescence and transformation are mediated by the ERK/mitogen-activated protein (MAP) kinase pathway.
The ERK pathway was shown to be responsible for senescence induced by oncogenic Ras and Raf (22, 47), and we have found that Pak4 levels increase in response to oncogenic Ras. We were therefore interested in determining whether the ERK pathway may be required in Pak4-induced senescence. We found that ERK activity was strongly stimulated in primary cells stably expressing Pak4 (Fig. 5A). Furthermore, treating cells with a chemical inhibitor of the MEK-ERK pathway partially abrogated Pak4-induced premature arrest in primary fibroblasts (Fig. 5B and C) and led to a decrease of about 50% in the number of SA-β-Gal-positive cells compared to what was seen in the absence of the drug (Fig. 5D and E). Interestingly, the flattened and enlarged shape typical of Pak4-infected cells did not seem to be affected by treatment with the MEK inhibitor. This suggests that the ERK pathway contributes to only some aspects of the senescent phenotype, which include SA-β-Gal expression, but it may not contribute to the changes in cell morphology induced by Pak4.
FIG. 5.
Pak4-induced cell cycle arrest and premature senescence are mediated by the ERK pathway. A. ERK kinase activity is elevated in primary MEFs infected with activated Pak4. ERK kinase activity was assayed in cell populations infected with empty vector (E.V.), activated PAK4 (PAK4*), or RasV12. ERK kinase activity was measured by immunocomplex kinase assay at day 2 postselection using MBP as a substrate. Levels of endogenous ERK were assessed by Western blotting using an anti-ERK antibody. B. Inhibition of MEK abrogates the growth inhibition triggered by Pak4. Growth curves of MEFs infected with empty vector (pLPC), activated PAK4 (PAK4*), or RasV12 are indicated. Cells were either treated with 50 μM of the MEK inhibitor PD 98059 (PD) or left untreated (NO). Open bars indicate empty vector, black bars indicate activated Pak4, and gray bars indicate activated Ras. d4 is day 4 postselection; d6 is day 6 postselection. For each condition, the percentage of cells relative to the empty vector control is indicated. C. Inhibition of MEK abrogates the effects of Pak4 on BrdU incorporation. The percentages of BrdU-incorporating cells at day 6 postselection in cell populations infected with empty vector, activated PAK4 (PAK4*), or RasV12 are shown (open bars indicate untreated cells; checked bars indicate cells treated with 50 μM PD98059). D and E. Inhibition of MEK abrogates the induction of SA-β-Gal expression in response to Pak4. Percentages of cells positive for SA-β-Gal (at pH 6.0) at day 4 (open bars) and at day 6 (closed bars) postselection are shown. NO is untreated; PD is PD98059-treated cells. Cells were scored as in Fig. 1F. Typical fields of cells are shown in panel E. SA-β-Gal-positive cells stain blue. F. Inhibition of MEK abrogates Pak4-induced transformation. NIH 3T3 cells stably transfected with either empty vector (E.V.) or activated PAK4 (PAK4*) were grown in soft agar for 2 weeks in the absence or presence of the indicated drugs. Colonies are shown at magnification ×4.
Interestingly, a MEK inhibitor also blocked Pak4-induced transformation in immortalized fibroblasts, while a p38 inhibitor had no effect (Fig. 5F). Our results suggest that Pak4-induced senescence as well as transformation are mediated by a pathway which operates through MEK and the ERK MAP kinase pathway.
Pak4 phosphorylates and activates Raf and activates the ERK pathway.
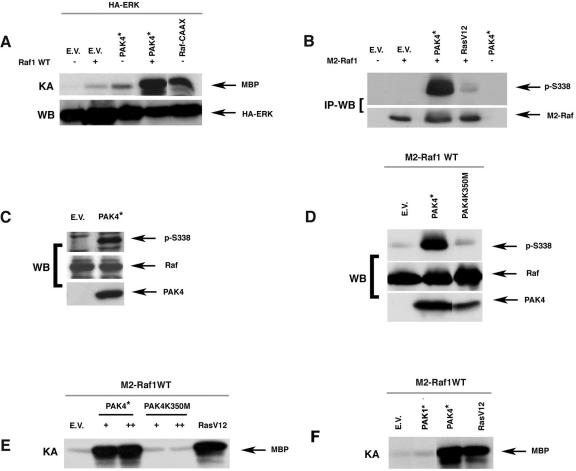
The results described above indicate that the ERK pathway has an important role in Pak4 signaling, leading both to transformation and premature senescence. Since Pak kinases have been shown to have a role in Raf activation (9, 14, 21), we hypothesized that Pak4 may activate the ERK pathway through Raf. The ERK pathway is readily activated in primary cells that are stably infected with Pak4 and undergoing senescence. However, in transiently transfected cells, Pak4 caused only a slight elevation of ERK activity, similar to wild-type Raf1 (Fig. 6A). However, when coexpressed, Pak4 and Raf strongly synergized to give a level of ERK activation comparable to what was seen with activated membrane-targeted Raf (RafCAAX) (Fig. 6A). Furthermore, Pak4 was able to induce phosphorylation of overexpressed and endogenous Raf on serine 338 in vivo (Fig. 6B and C, respectively), and it directly phosphorylated Raf on this site in vitro (Fig. 6D). In vitro kinase assays indicated that Pak4 could also stimulate Raf kinase activity (Fig. 6E). In contrast, although Pak1 was previously shown to affect Raf activity (9, 14), we found that compared with Pak4, Pak1 had very little affect on Raf activity (Fig. 6F). These results suggest that Pak4 activates Raf via phosphorylation and that this activation may play an important role in the activation of the ERK pathway. In addition, our results suggest that the ERK pathway plays a key role in Pak4-induced cell growth regulation.
FIG. 6.
PAK4 cooperates with Raf to activate the ERK pathway and induces Raf kinase activation. A. Pak4 cooperates with Raf to activate the ERK pathway. 293 cells were transfected with hemagglutinin (HA)-ERK with or without wild-type Raf1 (Raf1 WT) along with the indicated vectors. ERK activity was determined by immunocomplex kinase assay using MBP as a substrate. Levels of exogenous ERK expression were assessed by Western blotting with an antibody against the hemagglutinin epitope. B. Pak4 leads to in vivo Raf phosphorylation. 293 cells were transfected with M2-tagged Raf1 with or without activated PAK4 (PAK4*) or RasV12. Exogenous Raf was immunoprecipitated from cell lysates with an anti-M2 antibody, and Raf protein levels were visualized by Western blotting using an anti-M2 antibody (bottom). The same Western blots were also probed with antibodies specific for phosphoserine-338 (p-S338) (top). C. Pak4 leads to phosphorylation of endogenous Raf. Protein extracts from NIH 3T3 cells stably expressing empty vector (E.V.) or activated Myc-tagged PAK4 (PAK4*) were analyzed for endogenous Raf1 expression by Western blotting using an anti-Raf antibody. The same blots were probed with antibody directed against Raf Ser-338 (pS338) to examine phosphorylated Raf levels. PAK4 expression was assessed by Western blotting using an anti-Myc antibody. D. Pak4 phosphorylates Raf in vitro. Activated Myc-tagged PAK4 (PAK4*) or kinase-dead Myc-tagged PAK4 (PAK4K350M) were immunopurified from transfected 293 cell extracts using anti-Myc antibodies. Immunoprecipitates were mixed with Raf1 that had also been immunopurified from transiently transfected 293 cells. In vitro kinase assays were then carried out. Raf phosphorylation status was assessed by Western blotting using an anti-phosphoserine-338 antibody (p-S338). E. Pak4 increases Raf kinase activity. NIH 3T3 cells were transfected with empty vector (E.V.), activated PAK4 (PAK4*), kinase-dead PAK4 (PAK4K350M), or activated Ras (RasV12), along with M2-tagged Raf1 as indicated. Transfected Raf was immunoprecipitated from cell lysates with an M2 antibody, and Raf kinase assays were performed as detailed in Methods. MBP phosphorylation is shown. F. Pak4 activates Raf activity more efficiently than Pak1. Cells were transfected with activated Pak1, Pak4, or RasV12. Raf kinase activity was assessed as in panel E, described above.
DISCUSSION
The Rho GTPases and the Dbl family of guanine nucleotide exchange factors that activate them are thought to play key roles in the oncogenic process (36). In their constitutively active states, Dbl family members are potent oncogenes, found in several human cancers (8, 18). Cdc42, Rac, and Rho are required for transformation by Dbl family members and are each thought to contribute to different aspects of transformation (24). In order to understand how the Rho GTPases regulate growth and transformation, it is important to determine which target proteins mediate these processes. Pak4 is an effector protein for the Rho GTPase Cdc42 (1), and like activated Cdc42 (23, 24, 32), activated Pak4 promotes anchorage-independent growth in immortalized fibroblasts (35).
Often, oncogenes that transform immortalized cells lead to a very different outcome in primary cells. Expression of oncogenic Ras in primary fibroblasts, for example, leads to inhibition of cell growth and induction of premature cellular senescence (38). Other oncogenes, such as c-Myc, instead induce apoptosis (5). In both cases, these are thought to be defense mechanisms that the cell initiates to prevent the uncontrolled proliferation that would otherwise occur. In order to transform cells, oncogenes must ultimately be able to bypass the program leading to premature senescence. This program is presumably already compromised in cells that are predisposed for transformation or in immortalized cells, such as NIH 3T3 cells. This is most likely caused by genetic mutations that affect senescence pathways. Understanding the signaling pathways that control premature senescence is therefore crucial for understanding how oncogenes can promote uncontrolled cell growth and cancer and ultimately for developing treatments that will target the signaling pathways that are improperly regulated in cancer cells.
We have found that activated Pak4, although highly transforming in immortalized fibroblasts and overexpressed in cancer cell lines, caused primary fibroblasts to stop growing and to become senescent. Furthermore, Pak4 levels were upregulated in response to stimuli that are known to promote premature senescence, and the absence of Pak4 led to an increase in cell growth and a decrease in senescence. Cell cycle regulatory proteins, including p19ARF, p16INK4a, p21CIP, and p53, were upregulated in response to Pak4-induced senescence. Furthermore, in primary fibroblasts that lack p19ARF and p16INK4a, activated Pak4 no longer induced premature senescence. Instead, p19ARF/p16INK4a null primary fibroblasts continued to proliferate even after they were infected with activated Pak4. These results indicate that Pak4 plays a role in a signaling pathway leading to premature senescence and that either p19ARF or p16INK4a or both are required for this process.
In primary cells infected with activated Pak4, there was an increase in the amount of activated ERK MAP kinase, and inhibition of the ERK pathway abrogated PAK4-induced senescence. The ERK MAP kinase is known to regulate transcription factors, thereby controlling gene expression (45), and this may explain why it has an important role in regulating cell growth and senescence. In fact, p16INK4a expression can be directly regulated by transcription factors of the ETS family, which are in turn regulated by ERK (2, 26, 27), although the mechanism by which p19ARF may be regulated by the ERK pathway is still not clear.
We found that Pak4 directly phosphorylated Raf on serine 338, a phosphorylation site which is essential for Raf activation (12, 25), and considerably increased Raf kinase activity. Furthermore, we have found that Pak4 can cooperate with Raf to activate the ERK pathway. Raf activation generally requires membrane targeting in response to Ras, as well as phosphorylation by protein kinases (11). Our results suggest that Pak4 may be one of the kinases that can phosphorylate and activate Raf. Other Paks have also been shown to phosphorylate Raf and thereby lead to ERK activation (9, 14, 21). However, activated Pak4 stimulated Raf activity significantly more strongly than Pak1 (Fig. 6). Premature senescence in response to Pak4 seems to be quite specific, because activated Pak1 did not induce premature senescence. Taken together, these results suggest a correlation between strong Raf activation by Pak4 and the induction of premature senescence, further strengthening the idea that Raf plays a key role in the signaling pathway by which Pak4 induces premature senescence.
Our results indicate that the ERK pathway plays an important role downstream to Pak4 in the premature senescence pathway. While connections between Pak proteins and the Raf-ERK pathway have been previously illustrated, our results now suggest a physiological role for ERK activation downstream of Pak4. Our results are consistent with a model in which Pak4 leads to Raf phosphorylation and activation of the ERK pathway, which in primary cells leads to induction of genes such as p19ARF and p16INK4a. This in turn leads to p53 stabilization and regulation of retinoblastoma phosphorylation, resulting in premature senescence. The ERK pathway is strongly activated by Ras. Interestingly, we found that Ras did not promote senescence in Pak4 null MEFs. These results are complicated by the fact that Pak4 null cells also had a lower overall background level of senescence, yet they do suggest a role for Pak4 downstream to Ras and upstream to the ERK pathway during premature senescence.
In addition to changes in the expression of cell cycle regulatory proteins, cellular senescence is also characterized by profound changes in cell morphology, which are likely associated with cytoskeletal reorganization (44, 46). Little is known about the mechanisms responsible for these changes, but recent evidence points to a possible role for the actin-binding ERM proteins in the cell shape changes associated with senescence (46). It is interesting that Pak proteins are known to promote cytoskeletal reorganization (3). Pak4 in particular promotes profound cytoskeletal changes in immortalized cells (1), and we have found that primary MEFs expressing Pak4 show remarkable changes in cell shape as early as day 2 postselection. These include cell flattening and an abundance of polymerized actin at the edges of the cells. These morphological changes were much more pronounced in Pak4-infected cells than in Ras-infected cells and were apparent several days earlier in the Pak4-infected cells. It is interesting that the MEK inhibitor had a strong effect on SA-β-Gal expression in Pak4-expressing cells but did not have a dramatic effect on the cell shape changes triggered by Pak4. These results suggest that Pak4 could promote premature senescence by divergent pathways, which include the regulation of cell cycle regulatory proteins as well as alterations in cytoskeletal organization and morphology.
Rho GTPases and their effectors have long been associated with cell proliferation and transformation (36). However, this is the first study to show a role for a Rho GTPase effector protein in the signaling pathway leading to p19ARF and p16INK4a induction and premature senescence. Furthermore, this work provides important information about a new function of Pak4, which is the founding member of the group B family of Paks. We show that Pak4 is part of a family of proteins that can both transform cells and promote senescence, two apparently contradictory responses, depending on the cell type. Thus, in the presence of activated Pak4, the cell must make a decision whether to proliferate or senesce. This depends on the presence or absence of signaling pathways involved in the senescence process. Transformation by Pak4 presumably requires genetic mutations that inactivate the signaling pathways required for senescence. Immortalized cells, such as NIH 3T3 cells, are indeed known to have genetic mutations in genes encoding cell cycle inhibitory proteins. We would predict that cancer cells that have high levels of Pak4 also have mutations in genes in the senescence pathway, such as p53, thus allowing them to undergo transformation rather than senescence. By understanding the signaling pathways by which Pak4 may lead to the induction of premature senescence, we hope to be able to gain a better understanding of what goes wrong in cancer cells that are transformed in response to high levels of Pak4 or other transforming proteins.
Acknowledgments
This work was supported by a grant from the NIH (CA76342) to A.M.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani, R. M., A. Z. Young, and C. B. Shifflett. 2001. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc. Natl. Acad. Sci. USA 98:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagrodia, S., and R. A. Cerione. 1999. PAK to the future. Trends Cell Biol. 9:350-355. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, A. L., and A. Hall. 2000. Rho GTPase and their effector proteins. Biochem. J. 348:241-255. [PMC free article] [PubMed] [Google Scholar]
- 5.Bringold, F., and M. Serrano. 2000. Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol. 35:317-329. [DOI] [PubMed] [Google Scholar]
- 6.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277:550-558. [DOI] [PubMed] [Google Scholar]
- 7.Campisi, J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11:S27-S31. [DOI] [PubMed] [Google Scholar]
- 8.Cerione, R. A., and Y. Zheng. 1996. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 8:216-222. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary, A., W. G. King, M. D. Mattaliano, J. A. Frost, B. Diaz, D. K. Morrison, M. H. Cobb, M. S. Marshall, and J. S. Brugge. 2000. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10:551-554. [DOI] [PubMed] [Google Scholar]
- 10.Damalas, A., S. Kahan, M. Shtutman, A. Ben-Ze'ev, and M. Oren. 2001. Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 20:4912-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon, A. S., and W. Kolch. 2002. Untying the regulation of the Raf-1 kinase. Arch. Biochem. Biophys. 404:3-9. [DOI] [PubMed] [Google Scholar]
- 12.Diaz, B., D. Barnard, A. Filson, S. MacDonald, A. King, and M. Marshall. 1997. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol. 17:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost, J. A., H. Steen, P. Shapiro, T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family protein. EMBO J. 16:6426-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 16.Hart, M. J., A. Eva, T. Evans, S. A. Aaronson, and R. A. Cerione. 1991. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 354:311-314. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick, L. 1965. the limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614-636. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman, G. R., and R. A. Cerione. 2002. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 513:85-91. [DOI] [PubMed] [Google Scholar]
- 19.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 20.Khosravi-Far, R., P. A. Solski, G. J. Clark, M. S. Kinch, and C. J. Der. 1995. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 15:6443-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 22.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, R., S. Bagrodia, R. Cerione, and D. Manor. 1997. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 7:794-797. [DOI] [PubMed] [Google Scholar]
- 24.Lin, R., R. A. Cerione, and D. Manor. 1999. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J. Biol. Chem. 274:23633-23641. [DOI] [PubMed] [Google Scholar]
- 25.Mason, C. S., C. J. Springer, R. G. Cooper, G. Superti-Furga, C. J. Marshall, and R. Marais. 1999. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 18:2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickoloff, B. J., V. Chaturvedi, P. Bacon, J. Z. Qin, M. F. Denning, and M. O. Diaz. 2000. Id-1 delays senescence but does not immortalize keratinocytes. J. Biol. Chem. 275:27501-27504. [DOI] [PubMed] [Google Scholar]
- 27.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409:1067-1070. [DOI] [PubMed] [Google Scholar]
- 28.Olson, M. F., N. G. Pasteris, J. L. Gorski, and A. Hall. 1996. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr. Biol. 6:1628-1633. [DOI] [PubMed] [Google Scholar]
- 29.Palmero, I., C. Pantoja, and M. Serrano. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 395:125-126. [DOI] [PubMed] [Google Scholar]
- 30.Palmero, I., and M. Serrano. 2001. Induction of senescence by oncogenic Ras. Methods Enzymol. 333:247-256. [DOI] [PubMed] [Google Scholar]
- 31.Perona, R., P. Esteve, B. Jimenez, R. P. Ballestero, S. Ramon y Cajal, and J. C. Lacal. 1993. Tumorigenic activity of rho genes from Aplysia californica. Oncogene 8:1285-1292. [PubMed] [Google Scholar]
- 32.Qiu, R. G., A. Abo, F. McCormick, and M. Symons. 1997. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol. Cell. Biol. 17:3449-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu, R. G., J. Chen, D. Kirn, F. McCormick, and M. Symons. 1995. An essential role for Rac in Ras transformation. Nature 374:457-459. [DOI] [PubMed] [Google Scholar]
- 34.Qiu, R. G., J. Chen, F. McCormick, and M. Symons. 1995. A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. USA 92:11781-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Qu, J., X. Li, B. G. Novitch, Y. Zheng, M. Kohn, J.-M. Xie, S. Koznin, R. Brosnon, A. Beg, and A. Minden. 2003. The PAK kinase is essential for embryonic viability and for proper neuronal development. Mol. Cell. Biol. 23:7134-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahai, E., and C. J. Marshall. 2002. RHO-GTPases and cancer. Nat. Rev. Cancer 2:133-142. [DOI] [PubMed] [Google Scholar]
- 37.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 38.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 39.Shelton, D. N., E. Chang, P. S. Whittier, D. Choi, and W. D. Funk. 1999. Microarray analysis of replicative senescence. Curr. Biol. 9:939-945. [DOI] [PubMed] [Google Scholar]
- 40.Sherr, C. J. 2001. Parsing Ink4a/Arf: “pure” p16-null mice. Cell 106:531-534. [DOI] [PubMed] [Google Scholar]
- 41.Tang, Y., Z. Chen, D. Ambrose, J. Liu, J. B. Gibbs, J. Chernoff, and J. Field. 1997. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol. Cell. Biol. 17:4454-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang, Y., S. Marwaha, L. Rutkowski, G. I. Tennekoon, P. C. Phillips, and J. Field. 1998. A role for Pak protein kinases in Schwann cell transformation. Proc. Natl. Acad. Sci. USA 95:5139-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang, Y., H. Zhou, A. Chen, R. N. Pittman, and J. Field. 2000. The Akt proto-oncogene links Ras to Pak and cell survival signals. J. Biol. Chem. 275:9106-9109. [DOI] [PubMed] [Google Scholar]
- 44.Thomas, D. M., H. S. Yang, K. Alexander, and P. W. Hinds. 2003. Role of the retinoblastoma protein in differentiation and senescence. Cancer Biol. Ther. 2:124-130. [PubMed] [Google Scholar]
- 45.Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205-215. [DOI] [PubMed] [Google Scholar]
- 46.Yang, H. S., and P. W. Hinds. 2003. Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol. Cell 11:1163-1176. [DOI] [PubMed] [Google Scholar]
- 47.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]