Abstract
Death receptor-mediated apoptosis is potently inhibited by viral FLIP (FLICE/caspase 8 inhibitory protein), which is composed of two tandemly repeated death effector domains (DEDs), through reduced activation of procaspase 8. Here, we show that equine herpesvirus 2-encoded viral FLIP E8 enhances Wnt/β-catenin signaling in a variety of cell lines. E8 was shown to strikingly augment Wnt3a signaling, as shown both in a luciferase assay for T-cell factor/β-catenin and through induction of endogenous cyclin D1. The effect of E8 was independent of its direct binding activity with DED-containing signaling molecules, including caspase 8 and FADD, in death receptor-mediated apoptosis. E8 enhanced Wnt signaling downstream of stabilized β-catenin, while a long form of cellular FLIP (c-FLIPL) enhanced stabilization of β-catenin in 293T cells. Consequently, coexpression of E8 and c-FLIPL synergistically increased Wnt signaling in 293T cells. Moreover, E8-mediated stimulation of Wnt signaling induced dramatic growth retardation in untransformed cell lines but not in transformed cell lines. Thus, viral FLIP E8 not only inhibits death receptor-mediated apoptosis but also enhances Wnt signaling pathways that are closely related to those of both ontogenesis and oncogenesis.
The death effector domain (DED) was initially identified in signaling molecules involved in apoptosis induced via death receptors such as Fas. Fas, a member of the tumor necrosis factor receptor superfamily, is responsible for apoptosis-inducing signals (20, 57) and plays an important role in the elimination of autoreactive lymphocytes, as well as tumor and virus-infected cells (34). Fas-induced apoptosis is mediated by recruitment of an adaptor molecule, Fas-associated death domain (FADD), to the DD of Fas (10). FADD, which is composed of a DD and a DED, then recruits procaspase 8/FLICE (FADD-like interleukin-1β-converting enzyme) by homophilic interaction of DEDs between FADD and procaspase 8, which comprises two tandemly repeated DEDs and a protease domain at its N- and C-terminal regions, respectively (7, 33). The complex constituted by at least Fas, FADD, and caspase 8 has been designated the death-inducing signaling complex (DISC), and proximity-induced cleavage/activation of caspase 8 is induced in the DISC (31). Fas-induced activation of caspase 8 can be inhibited by the cellular FLICE-inhibitory protein (c-FLIP), for which two splice variants, the long and short forms, are known (50). Both these variants contain two DEDs; the long form of c-FLIP (c-FLIPL) is homologous to procaspase 8 and has two tandemly repeated DEDs and a protease-like domain but no protease activity, while the short form of c-FLIP (c-FLIPS) is composed of only two DEDs.
A number of viruses encode viral FLIP (v-FLIP), which contains two tandemly repeated DEDs only (6, 18, 50). v-FLIP robustly blocks the Fas-mediated apoptosis-inducing signal by forming homophilic interactions with DEDs of FADD and/or caspase 8 (6, 18, 49) and has been considered to play a role in allowing virally infected cells to escape immune surveillance, since this utilizes Fas-induced apoptosis. Hence, the function of v-FLIP may be to allow some viruses to continue to proliferate, by enhancing the survival of virus-infected host cells. A number of viruses have been reported to encode v-FLIP, including E2 (bovine herpesvirus 4) (18, 49), E8 (equine herpesvirus 2) (6, 18, 49), ORF16 (saimirine herpesvirus) (49), K13/ORF71 (human herpesvirus 8) (18, 49), and MC159 (molluscum contagiosum poxvirus) (6, 18, 49), and all these viruses, other than MC159, are products of gammaherpesvirus.
Some recent evidence has suggested that FLIP exhibits a regulatory function for cell proliferation and differentiation, in addition to an inhibitory activity against Fas-induced apoptosis. c-FLIPL has been reported to promote activation of nuclear factor κB (NF-κB) and extracellular signal-regulated kinase (22). v-FLIP K13 was shown to activate the canonical and alternative NF-κB pathways, but other v-FLIPs do not share such activity (9, 30). K13 was also reported to activate the c-jun N-terminal protein kinase pathway (2), and, furthermore, to induce cellular survival, proliferation, and transformation through transcriptional upregulation of antiapoptotic and growth-promoting effector molecules through the activation of NF-κB, while neither E8 nor MC159 exhibits these biological activities (12, 15, 30, 46).
We have previously reported that transgenic (Tg) mice expressing v-FLIP E8 in thymocytes exhibit thymic atrophy arising from a reduction of thymocyte number (36). The thymic obsolescence might be a result of E8-induced suppression of T-cell development in the early stage of the transition from the double negative 3 (DN3) to the DN4 stage (36), where the number of thymocytes is known to increase. Interestingly, thymocytes of the Tg mice retained their ability to proliferate in response to in vitro stimulation with anti-CD3 and anti-CD28 monoclonal antibodies (MAbs). Taking these observations together, we concluded that E8 expressed in thymocytes acts as a negative regulator for their differentiation and/or proliferation in vivo, although the complete molecular mechanism underlying the function of E8 remains to be determined.
Wnt signaling is thought to be important for embryogenesis and ontogenesis (29) and has been reported to play an essential role in the differentiation of immature thymocytes from the DN to the double positive (DP) stage (14, 45). Wnt signaling is triggered by the binding of Wnt family ligands to their receptors, which are referred to as the Frizzled receptor family, and then transmitted to the Dishevelled protein, which inactivates glycogen synthase kinase-3β (GSK-3β) (55). β-Catenin is usually phosphorylated by GSK-3β before undergoing degradation through the ubiquitin-proteasome pathway (1). Wnt-induced inactivation of GSK-3β stabilizes β-catenin, causing its accumulation in the cytosol and subsequent transfer into the nucleus, where it binds to T-cell factor (TCF) family transcription factors, inducing the expression of Wnt-specific target genes that regulate cellular proliferation and/or differentiation (8, 24, 37). Staal et al. have reported that Wnt signaling is involved in the development of immature thymocytes from DN to DP, in which TCF-1 and lymphocyte enhancer factor 1 (LEF-1) are required to induce differentiation (45). Gounari et al. have studied Tg mice with stabilized β-catenin in thymocytes and showed that the total cell numbers in the thymus decreased and that differentiation was suppressed between the DN3 and DN4 stages (14).
Tg mice with stabilized β-catenin in thymocytes demonstrated a similar phenotype to that in our E8 Tg mice (36). For this reason, we hypothesized that E8 might act in the Wnt signaling pathway. In this study, E8 was shown to dramatically enhance Wnt signaling in various cell lines downstream of Wnt-induced stabilization of β-catenin. In addition, marked enhancement of Wnt signaling by expression of v-FLIP E8 allowed the detection of Wnt signaling that induced dramatic growth retardation in untransformed cell lines but not in transformed cell lines in vitro.
MATERIALS AND METHODS
Plasmid construction.
E8 and MC159 were expressed using a pME18s vector with a FLAG tag, as described previously (36, 51). To clone a full-length K13 cDNA, Hirt DNA from human herpesvirus 8-infected cells, which were kindly provided by K. Ueda (Osaka University, Japan), was used for PCR with Pfu polymerase. The FLAG-K13 expression plasmid was generated by ligating the K13 cDNA into pME18s with the FLAG tag. The reporter plasmid for NF-κB (pκB-luc) and its control reporter plasmid without the κB enhancer sequence (pdN-luc) were gifts from J. Fujisawa (Kansai Medical College, Osaka, Japan). The TCF-luciferase reporter plasmid (pTCFwt-luc) with seven tandem repeats of the TCF response element and a mutant reporter plasmid (pTCFmt-luc) (52) were kindly provided by M. Hijikata and K. Shimotohno (Kyoto University). pRL-TK, used for the internal control in the dual luciferase assay, was purchased from Promega. The dominant negative mutant of TCF4E (TCF4E N80), which lacks a DNA-binding domain, ICAT, and constitutively stabilized β-catenin, in which Ser33 was replaced by Tyr (β-catenin S33Y), were expressed as the vectors pMKITNeo-FLAG-N80 (40), pcDNA3.1-FLAG-ICAT (47), and pMKITNeo-β-catenin S33Y (25), respectively. The expression vectors for human c-FLIP and green fluorescent protein (GFP) were kindly provided by K. K. Lee (Kyoto University) and K. Umezono (Kyoto University), respectively.
Preparation of Wnt3a-conditioned medium.
Conditioned medium containing Wnt3a and control medium were prepared by cultivation of L cells stably expressing soluble Wnt3a (Wnt3a/L) and control cells (neo/L), respectively, as described previously (41).
Cell culture and transfection.
BALB/3T3, Rat-1, 293, 293T, KB, U2OS, wild-type mouse embryonic fibroblast (MEF), caspase 8−/− MEF (39), and FL cells were cultured in Dulbecco's modified Eagle's medium (Nacalai Tesque Inc.) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. Cells in six-well plates or 10-cm dishes were transfected with 1 μg/well or 4 μg/dish of expression vectors, respectively, using Lipofectamine Plus reagent (Invitrogen), in accordance with the manufacturer's instructions.
Quantification of cell viability.
KB cells in 6-well plates transfected with expression vectors for v-FLIP (0.8 μg/well) and GFP (0.2 μg/well) were seeded in 96-well plates. After 2 days of cultivation, cells were stimulated with 500 ng/ml of anti-Fas antibody, CH-11, together with 1 μg/ml of cycloheximide. To quantify cell viability in the 96-well plates, the number of GFP-positive cells per field of an immunofluorescence microscope were counted after 2 days of cultivation, as described previously (23).
Dual luciferase assay.
Cells in six-well culture plates were transfected with 1 μg/well pME18s, encoding v-FLIP, and 0.2 μg/well pTCFwt-luc or pTCFmt-luc, together with 0.02 μg/well pTK-RL. The total amount of DNA for each transfection was adjusted to 1.22 μg/well by adding empty vectors. Three hours after transfection, cells were reseeded in 48-well tissue culture plates, and cultured for around 18 h. Cells were then treated with conditioned medium containing soluble Wnt3a (Wnt3a/L-CM) or control medium (neo/L-CM) for 12 to 24 h, and a luciferase assay was performed using a dual luciferase assay kit (Promega), in accordance with the manufacturer's instructions. All data were expressed as means ± standard deviations (SD) (n = 3).
Concentration of cells expressing the exogenous gene.
A MACS system (Miltenyi Biotec) was utilized to concentrate cells expressing the exogenous gene. A truncated form of the mouse interleukin-3 receptor β-chain, tAic-2A (21), was used as a marker; this protein does not introduce any signaling in the cells in which it is expressed, because it lacks an intercellular domain. BALB/3T3 cells were transfected with an expression vector for E8 or an empty vector, together with vectors for tAic-2A and GFP. After treatment with Wnt3a/L-CM or neo/L-CM, cells were incubated with rat anti-Aic-2 MAb (58) followed by magnetic-bead-labeled mouse anti-rat immunoglobulin G Ab (Miltenyi Biotec) and then applied to a MidiMACS MS separation column according to the manufacturer's instructions.
Western blotting.
Cells were lysed in ice-cold lysis buffer (20 mM Tris HCl, pH 7.4, containing 10% glycerol, 1% Triton X-100, 150 mM NaCl, and 1 mM EDTA) with protease inhibitor cocktail tablets (Roche). The lysate was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting, as previously described (27).
Colony formation assay.
Cells in 6-well plates were cotransfected with 0.9 μg/well and 0.1 μg/well of the expression vectors for E8 and a neomycin resistance gene, respectively, and seeded in 24-, 12-, or 6-well plates immediately after transfection. Cells were then cultured with Wnt3a/L-CM or neo/L-CM, both of which contained 0.5 to 1.0 mg/ml of G418 (Nacalai Tesque Inc.), for 10 to 21 days. The culture medium was replaced every 2 days with fresh Wnt3a/L-CM or neo/L-CM containing G418. Colonies formed as G418-resistant cells were stained with Giemsa staining solution, or the cell number was determined after suspension following treatment with trypsin.
RESULTS
v-FLIP E8 enhances Wnt3a-induced signaling.
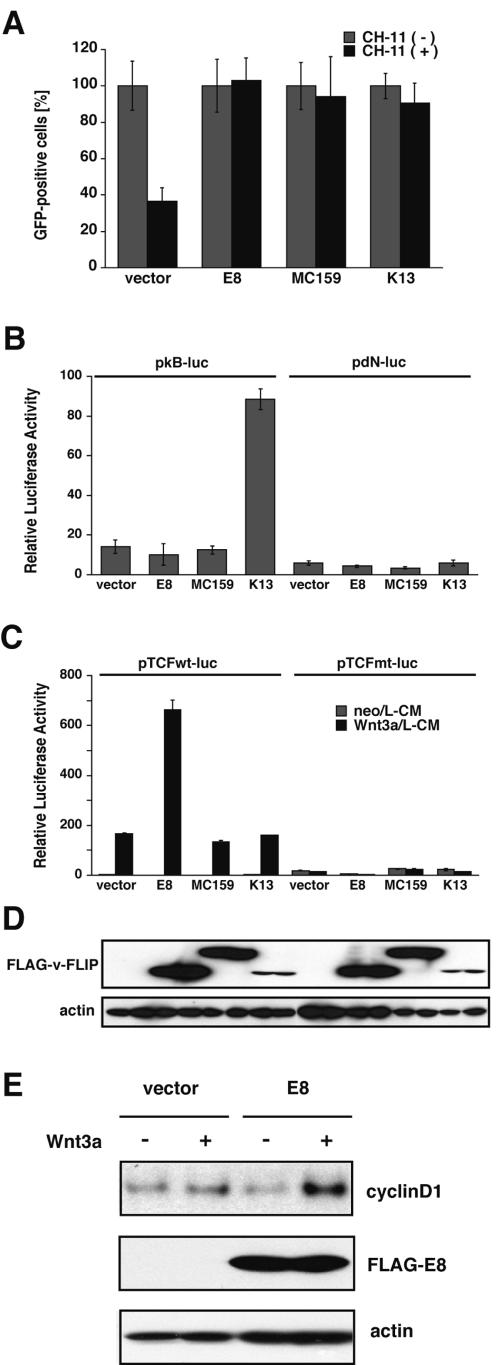
We first examined various functions of three kinds of v-FLIP: equine herpesvirus 2-encoded E8, human herpesvirus 8-encoded K13, and human molluscum contagiosum poxvirus-encoded MC159. All the v-FLIPs strongly inhibited Fas-induced apoptosis upstream of caspase 3 activation in KB cells (Fig. 1A ; see Fig. S1 in the supplemental material). In addition, K13 showed a potent ability to activate NF-κB in BALB/3T3 cells, while the other two v-FLIPs, expression levels of which were much higher than that of K13 (Fig. 1D), did not activate NF-κB (Fig. 1B), as previously reported (9, 12, 28).
FIG.1.
Viral FLIP exhibits a wide variety of biological activities, including enhancement of Wnt3a signaling. (A) KB cells transfected with an expression plasmid of the indicated v-FLIP together with that of GFP were plated in 96-well plates. After a 2-day cultivation, cells were stimulated with an agonistic anti-human Fas MAb, CH-11, to induce Fas-mediated apoptosis. After another 2 days of cultivation, the number of GFP-positive cells per field of a microscope was counted. Cell viability is represented as the percentage of GFP-positive cells. Data are expressed as means ± SD (n = 3). (B) BALB/3T3 cells were transfected with an expression plasmid of the indicated v-FLIP, together with reporter plasmids containing the NF-κB-responsive element firefly luciferase (pkB-luc) or control luciferase (pdN-luc) in addition to pTK-Renilla luciferase as an internal control. After a 30-h cultivation, a dual luciferase assay was performed. Results are expressed as means ± SD (n = 3). The data are representative of two independent experiments that gave similar results. (C) BALB/3T3 cells were transfected with an expression plasmid for v-FLIP, together with pTCFwt-luc or pTCFmt-luc and pTK-Renilla luciferase. After an 18-h cultivation, cells were stimulated with Wnt3a/L-CM or neo/L-CM for 12 h, and then a dual luciferase assay was performed. The data are representative of more than five independent experiments that gave similar results. (D) The expression level of FLAG-tagged v-FLIPs in the transfected BALB/3T3 cells (B and C) was assessed by Western blotting with anti-FLAG Ab. β-Actin was also detected as a loading control. (E) BALB/3T3 cells transiently expressing E8 together with truncated Aic-2A were concentrated as described in Materials and Methods. After cultivation with Wnt3a/L-CM or neo/L-CM for 12 h, concentrated cells expressing exogenous genes were analyzed by immunoblotting with anti-cyclin D1, anti-FLAG, and antiactin Abs.
In Tg mice expressing v-FLIP E8 in thymocytes, we observed the thymocytes not only to be resistant to Fas-induced apoptosis but also to be defective in in vivo development and/or proliferation (36), suggesting that v-FLIP E8 regulates an unidentified signal involved in growth and/or differentiation of immature thymocytes in vivo. We then examined the effect of E8 on Wnt signaling, which is reported to be involved in the differentiation of thymocytes in vivo (14, 45). A dual luciferase assay was performed to quantify the Wnt3a-induced transcription activity of TCF. Mouse BALB/3T3 cells were transfected with an expression plasmid encoding v-FLIP and were then cultured with either Wnt3a/L-CM or neo/L-CM (41). BALB/3T3 cells, which express an undetectable amount of c-FLIP by Northern hybridization (23), were shown to be sensitive to stimulation with Wnt3a, and expression of E8 definitively increased Wnt3a-induced transcriptional activity (Fig. 1C). The stimulatory effect of E8 was further examined by performing Western blotting to quantify Wnt3a-induced expression of endogenous cyclin D1 in BALB/3T3 cells (Fig. 1E). Expression of cyclin D1, which is the product of one of the target genes of the Wnt/β-catenin signaling pathway (42, 48), was potently upregulated in cells expressing E8 after treatment with Wnt3a. However, K13 did not exhibit significant Wnt signal-enhancing activity. Since its expression was much less than that of E8 (Fig. 1D), there remains a possibility that K13 has the potential to enhance Wnt signal. Another v-FLIP, MC159, which is derived from poxvirus, failed to enhance Wnt signaling in spite of its high expression. Taken together, these results show that expression of gammaherpesvirus-encoded v-FLIPs E8 and K13 dramatically enhances Wnt signaling-induced and NF-κB-induced transcriptional activity, respectively, but that MC159, which is derived from poxvirus, failed to either activate NF-κB or enhance Wnt signaling.
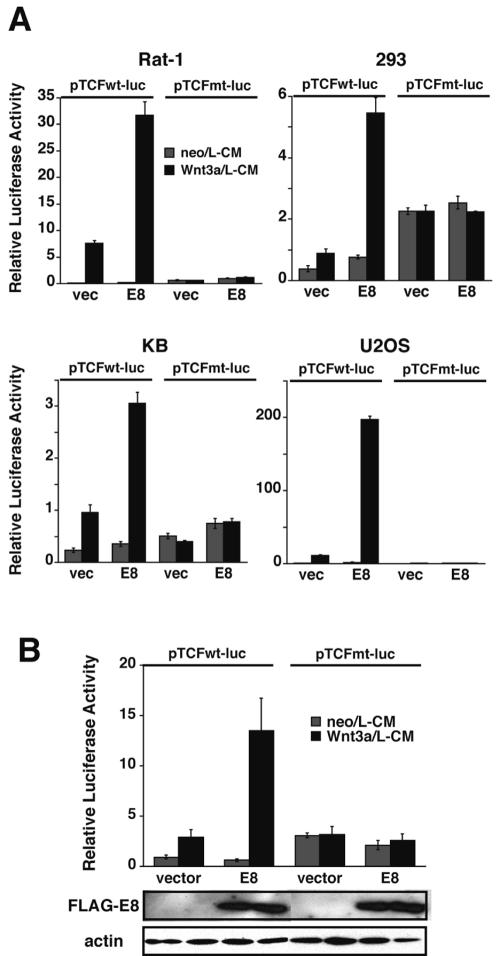
We next analyzed whether the Wnt signal-enhancing activity of E8 is generally observed in a wide variety of cell lines (Fig. 2A). We examined Rat-1, 293, 293T, KB, U2OS, and MEF cells, which are sensitive to stimulation by Wnt3a. All the cells examined showed similar sensitivities to E8-mediated enhancement of Wnt3a-induced signaling.
FIG. 2.
E8 enhancement of Wnt signaling is observed in various cell lines. (A) Wnt signaling in the presence of E8 was examined in Rat-1, 293, KB, and U2OS cells, as described in the legend to Fig. 1C. All the data are representative of three independent experiments that gave similar results. (B) A dual luciferase assay for Wnt signaling was carried out in the same manner as for panel A, using caspase 8−/− MEF cells. The results are representative of those obtained from more than three independent experiments that gave similar results. The expression level of E8 was confirmed by Western blotting, as for Fig. 1C. vec, empty vector.
FADD and caspase 8 are dispensable for E8-mediated enhancement of Wnt signaling.
E8, which is composed of two tandemly repeated DEDs, has been reported to interact homophilically with the DED of caspase 8 and/or FADD (6, 18, 49). We therefore analyzed whether caspase 8 and FADD are necessary for E8-mediated enhancement of Wnt3a-induced signaling, using caspase 8 knockout and FADD knockout MEFs. Figure 2B clearly shows that Wnt signal enhancement by transiently expressed E8 did not change in caspase 8-deficient cells. We also examined the effect of E8 on Wnt signaling in FADD knockout MEFs (3, 56) and found that E8 can enhance Wnt3a signaling in these cells (see Fig. S2 in the supplemental material). Thus, FADD and caspase 8 were shown to be dispensable for E8-mediated enhancement of Wnt3a-induced signaling.
E8 enhances the Wnt-induced canonical signaling pathway.
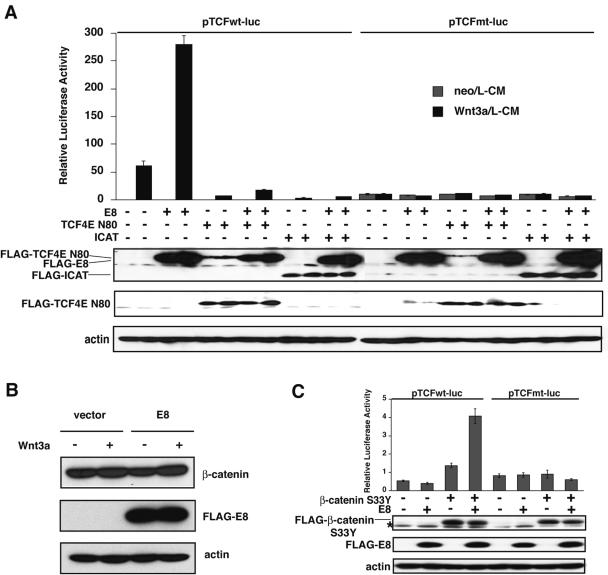
Transcriptional activity of TCF is usually suppressed in the absence of Wnt signals (48). Once Wnt signaling begins, stabilization of β-catenin is induced, and the stabilized β-catenin forms a complex with TCF in the nucleus, with the complex subsequently inducing Wnt-specific transcription. To verify whether E8 stimulates this Wnt/β-catenin signal, which is referred to as the canonical Wnt signal, we tested the effects of two inhibitory proteins which directly suppress the association of β-catenin and TCF on E8-induced stimulation of Wnt signaling. One is a β-catenin-binding protein called ICAT, which blocks the interaction between β-catenin and TCF (47). The other is a dominant negative mutant of TCF4E, termed TCF4E N80, which lacks the DNA binding region of TCF4E (26, 40). TCF4E is a member of the TCF/LEF family and may act downstream of Wnt3a, especially in fibroblasts (17, 38). We analyzed whether these inhibitory molecules suppressed the E8-induced activation of TCF-directed luciferase activity after cultivation with or without Wnt3a in BALB/3T3 cells. As shown in Fig. 3A, both TCF4E N80 and ICAT strongly inhibited Wnt3a-induced transcription in cells transfected either with or without the E8 expression vector. Thus, the interaction of β-catenin and TCF plays an essential role in E8-mediated intensification of Wnt3a-induced signaling.
FIG. 3.
E8 enhances the Wnt canonical signaling pathway downstream of stabilized β-catenin. (A) BALB/3T3 cells were transfected with an E8 expression plasmid and with or without dominant negative TCF4E (TCF4E N80) or the ICAT vector. After an 18-h cultivation, cells were treated with or without Wnt3a for 12 h, and then luciferase activity was quantified. Expression levels of transiently expressed FLAG-tagged E8, TCF4E N80, and ICAT were determined by Western blotting with anti-FLAG Ab and anti-TCF Ab. (B) 293 cells were transfected with an empty vector or an E8 expression plasmid. After an 18-h cultivation, cells were treated with or without Wnt3a for 12 h, and then expression levels of endogenous β-catenin and actin and exogenous E8 were analyzed by immunoblotting with anti-β-catenin Ab, antiactin Ab, and anti-FLAG Ab, respectively. (C) BALB/3T3 cells were cotransfected with an E8 expression vector and a vector for constitutively stabilized β-catenin (β-catenin S33Y). Cells were cultured with or without Wnt3a for 30 h, and then luciferase activity was determined. The dose of plasmids in each sample was equalized with that of empty vectors. All the data shown are representative of three independent experiments that gave similar results. Expression levels of transiently expressed FLAG-tagged E8 and β-catenin S33Y were determined by Western blotting with anti-FLAG Ab. The nonspecific band is indicated by an asterisk.
E8 enhances the Wnt canonical signaling pathway downstream of stabilized β-catenin.
Viral FLIP E8 was clearly shown to enhance the Wnt canonical signaling pathway, in which stabilized β-catenin plays an essential role. Therefore, we analyzed whether E8 enhances the stabilization of β-catenin. Although the stimulation of 293 cells with Wnt3a increased the expression level of β-catenin, E8 did not enhance the expression of β-catenin in the presence or absence of Wnt3a (Fig. 3B), indicating that E8 has no effect on the stabilization of β-catenin. Then we examined whether E8 enhances Wnt signaling downstream of the stabilization of β-catenin by using a mutant of β-catenin, β-catenin S33Y, which is reported to directly activate the transcription of TCF/LEF-1 (25). In β-catenin S33Y, replacement of Ser33 by Tyr causes the loss of a GSK-3β phosphorylation site (Ser33), resulting in acquisition of resistance to GSK-3β-directed phosphorylation-induced degradation (25). As expected, BALB/3T3 cells expressing β-catenin S33Y alone showed increased luciferase activity induced by the transactivation of TCF (Fig. 3C). Moreover, BALB/3T3 cells expressing E8 together with β-catenin S33Y clearly showed increased luciferase activity compared with that induced by β-catenin S33Y alone (Fig. 3C). Thus, all the results show that E8 stimulates the Wnt canonical pathway downstream of β-catenin stabilization and that the stimulatory effect of E8 on Wnt signaling is independent of the regulation of β-catenin degradation.
Comparison of Wnt signal-enhancing activities of v-FLIP E8 and c-FLIPL in 293T cells.
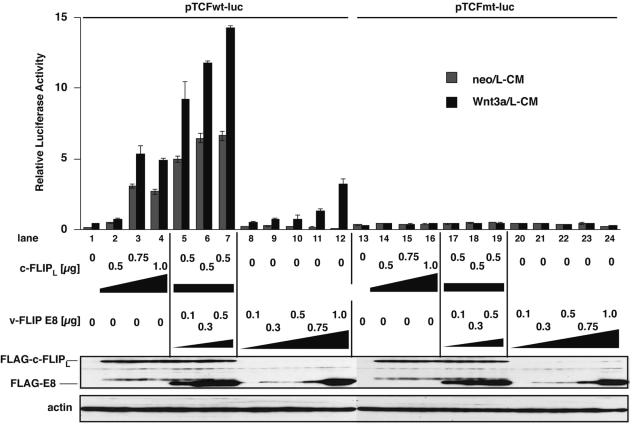
Although we tried to analyze the effects of c-FLIPS and c-FLIPL on Wnt signaling in BALB/3T3, KB, and other cell lines, it proved impossible to accurately assess the effect of c-FLIP because the expression of c-FLIP itself provoked apoptosis in these cells, as previously reported (13, 16, 19, 43). However, 293T cells appeared to be insensitive to c-FLIP-induced apoptosis, thereby allowing evaluation of c-FLIP activity on Wnt signaling in 293T cells. Recently, Naito et al. reported that c-FLIPL, but not c-FLIPS, promotes intracellular Wnt signaling in 293T cells by inducing the stabilization of β-catenin through inhibiting the ubiquitylation of β-catenin (35). We therefore compared the Wnt signal-enhancing activities of v-FLIP E8 and c-FLIPL in 293T cells. As recently reported, Wnt signaling was evoked by overexpression of c-FLIPL alone, regardless of Wnt3a stimulation, whereas E8 strengthened Wnt signaling only when cells were stimulated with Wnt3a, with the effect occurring in a dose-dependent manner (Fig. 4). c-FLIPL activates TCF-associated transcription in the absence of Wnt, while E8 enhances Wnt-induced signaling. In addition, coexpression of c-FLIPL and E8 was shown to synergistically enhance Wnt signaling. Putting these results together, it appears that c-FLIPL and E8 enhance the same Wnt canonical signaling pathway, but E8 and c-FLIPL enhance Wnt signaling downstream and mainly upstream, respectively, of stabilized β-catenin.
FIG. 4.
E8 and c-FLIPL enhance Wnt signaling by different molecular mechanisms in 293T cells. 293T cells were transfected with the indicated amounts of expression plasmids for c-FLIPL and E8, followed by stimulation with or without Wnt3a for 18 h, and then luciferase activity was quantified. The dose of plasmids in each sample was equalized with that of empty vectors. The data are representative of two independent experiments that gave similar results. Expression levels of c-FLIPL and E8 were determined by Western blotting with anti-FLAG Ab.
E8 has a different effect on the growth of transformed and untransformed cells by enhancing Wnt3a signaling.
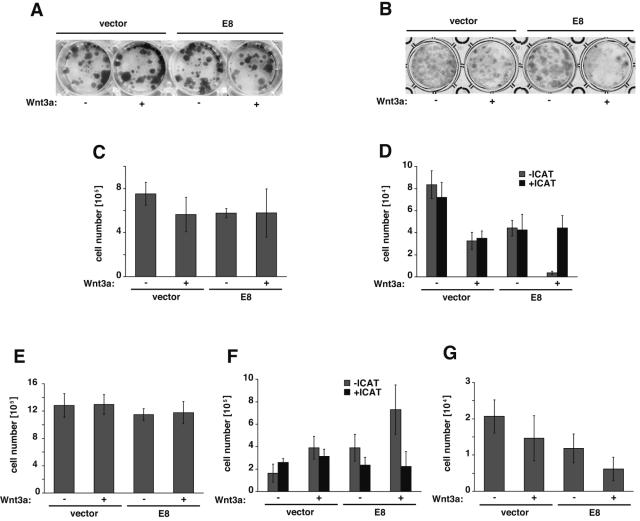
Wnt signaling has been reported to affect cell growth and differentiation in vivo, especially in embryogenesis, but these effects of Wnt signaling have not been clearly confirmed in in vitro experiments. We hypothesized that the in vitro effects of Wnt signaling on cell growth might be significantly detectable upon the enhancement of Wnt signaling by the expression of E8. We first analyzed the effect of Wnt3a, in the absence of E8, on the growth of a transformed epithelial cell line, KB, and an immortalized but nearly untransformed fibroblast cell line, BALB/3T3. Treatment with Wnt3a did not significantly affect the proliferation of KB cells, while BALB/3T3 cells showed inhibition of proliferation upon treatment with Wnt3a (see Fig. S3 in the supplemental material). We then examined the cooperative effects of Wnt3a and E8 on the growth of U2OS, KB, FL, wild-type MEF, and BALB/3T3 cells, all of which are sensitive to the E8-induced stimulation of Wnt signaling (Fig. 2; data not shown for FL cells). A colony formation assay was performed in E8-encoding plasmid-transfected cells to elucidate the physiological function of Wnt3a plus E8. In the colony formation assay, a neomycin-resistant gene was cotransfected with an E8 expression vector as a transfection marker to select cells expressing the transfected genes by cultivation with G418. Figure 5 and Fig. S3 in the supplemental material show Giemsa-stained colonies and cell numbers quantified after 2 or 3 weeks of cultivation of U2OS, KB, FL, wild-type MEF, and BALB/3T3 cells. As a result, U2OS and FL cells expressing E8 did not show any significant effects on their growth in the presence or absence of Wnt3a, but KB cells expressing E8 showed enhanced growth in the presence of Wnt3a. On the contrary, Wnt3a potently inhibited the growth of BALB/3T3 and MEF cells expressing E8. The opposite effects of E8 plus Wnt3a in both KB and BALB/3T3 cells were cancelled by expression of ICAT, which inhibits Wnt signaling by directly suppressing the binding of β-catenin to TCF. All these results indicate that E8 inhibits proliferation of untransformed cells (3T3 and MEF cells) by enhancing Wnt3a signaling but does not show such an inhibitory effect on transformed cells (U2OS, KB, and FL cells).
FIG. 5.
Enhanced Wnt3a signaling shows growth retardation in untransformed cells expressing E8. U2OS cells (A and C), BALB/3T3 cells (B), FL cells (E), and MEF (G) in six-well plates were transfected with an E8 expression plasmid or an empty vector together with a neomycin resistance gene, as described in Materials and Methods. BALB/3T3 cells (D) and KB cells (F) were cotransfected with the expression plasmids for E8 and ICAT together with a neomycin resistance gene. Cells were seeded in 12-well plates (A, C, E-G) or 24-well plates (B, D) at appropriate densities and cultured with Wnt3a/L-CM or neo/L-CM containing 1.0 mg/ml (A, C, E), 0.8 mg/ml (F, G) or 0.5 mg/ml (B, D) of G418 for 14 days (A-E) or 21 days (F, G). Colonies were stained with Giemsa staining solution (A, B), or the number of cells/well was quantified using a hemacytometer (C-G). The bars represent means ± SD (n = 3 to 6). All the data are representative of more than three independent experiments that gave similar results.
DISCUSSION
In this study, equine herpesvirus-encoded v-FLIP E8, which has been shown to inhibit death receptor-induced apoptosis, was shown to strikingly enhance Wnt signaling, which is known to play an important role in cell proliferation and differentiation. Since E8 Tg mice showed interruption of differentiation and/or proliferation of immature thymocytes (36), we assumed that E8 must possess other important biological activities, in addition to inhibitory activity for death receptor-mediated apoptosis. Because Wnt signaling is reported to be necessary for immature DN thymocytes to differentiate into the DP stage, and E8 Tg mice showed similar phenotypes to those in previously reported Tg mice with stabilized β-catenin (14), we hypothesized that E8 may have effects on Wnt signaling. Here, we have shown that transiently expressed E8 strongly enhances Wnt3a-induced signaling in a dual luciferase assay (Fig. 1C), as well as by detecting Wnt3a-induced expression of endogenous cyclin D1 (Fig. 1E). Such a Wnt-enhancing effect of E8 was shown to be general, since we observed the same effect in various cell lines, from rodent cells to human cells sensitive to Wnt3a (Fig. 2A).
Human Kaposi's sarcoma-associated herpesvirus-encoded v-FLIP K13 did not show an enhancement of Wnt3a-induced signaling similar to that of E8 (Fig. 1C). Since its expression was much less than that of E8 (Fig. 1D), K13 may have the potential to enhance Wnt signaling. However, molluscum contagiosum poxvirus-encoded MC159, which, similarly to E8 and K13, is composed of two tandemly repeated DEDs, did not enhance Wnt3a signaling (Fig. 1C). This result is consistent with the phenotype of Tg mice expressing MC159 in T cells. These mice show a drop in total thymocyte yield much smaller than that in E8 Tg mice (54). We speculate that MC159 might be unable to bind to a putative E8 target molecule that plays an essential role in E8-induced stimulation of Wnt signaling. This hypothesis is consistent with previously reported differences between MC159 and E8 in binding to other molecules containing DEDs (6, 18).
When v-FLIP functions as an inhibitor of Fas-mediated apoptosis, v-FLIP binds to FADD and/or caspase 8 by homophilic interaction through their DEDs (6, 18, 49). v-FLIP has been reported to prevent proximity-induced autoactivation of caspase 8 in the so-called DISC, which is constituted by at least Fas, FADD, and caspase 8, by inhibiting the recruitment of caspase 8 into the DISC (5). However, these interactions were unnecessary in E8's enhancement of Wnt3a signaling, because this enhancement was observed in both FADD-deficient and caspase 8-deficient MEF cells (Fig. 2B and Fig. S2 in the supplemental material). Consequently, we conclude that v-FLIP must enhance Wnt signaling by binding to molecules other than FADD or caspase 8, using its DED, since E8 contains only two DEDs which are known to interact homophilically with DEDs of other molecules. Therefore, E8 may stimulate Wnt3a-induced signaling by interacting with an unidentified DED-containing protein(s), and identification of the target molecule(s) of E8 will be of importance to clarify the molecular mechanism of the Wnt signal-enhancing activity of E8.
Our results suggest that E8 enhances Wnt3a-induced signaling promoted by the interaction between β-catenin and TCF (Fig. 3A). Wnt signaling is considered to be transduced not only by the so-called canonical pathway, which is activated by stabilized β-catenin, but also by a noncanonical pathway independent of β-catenin, such as a Ca2+-dependent pathway (44). Although we cannot completely eliminate the possibility that pathways other than the canonical pathway are involved in E8-induced enhancement of Wnt signaling, it is assumed that E8 stimulates the Wnt canonical pathway, since E8 also enhanced the transcriptional activity of the complex of TCF and the mutant β-catenin without stimulation by Wnt (Fig. 3C). In the Wnt canonical pathway, stabilized β-catenin has been shown to migrate into the nucleus and then form complexes with TCF/LEF (4, 32, 53). We examined the interaction of E8 and β-catenin or TCF using a coimmunoprecipitation assay in 293T cells overexpressing E8 and β-catenin or TCF. To date, we have been unable to detect an association of E8 with either TCF or β-catenin under conditions in which interaction between TCF and β-catenin was detected (data not shown). Thus, E8 may perform a function on Wnt signaling downstream of the stabilization of β-catenin, without directly binding to β-catenin or TCF.
Recently, Naito et al. reported that c-FLIPL could mimic Wnt-induced signaling in 293T cells, in cooperation with FADD, by preventing ubiquitylation and the subsequent degradation of β-catenin (35). Although we tried to confirm these results, we were unable to obtain the same results in various cell lines in which E8 enhancement of Wnt signaling was detected. However, we were able to detect c-FLIPL-directed mimicking of Wnt signaling activity only in 293T cells (Fig. 4). c-FLIPL was shown to activate the Wnt signal by itself, while E8 increased the intensity of Wnt signaling only following initial stimulation of the Wnt signal. Moreover, E8 was indicated not only to function downstream of stabilized β-catenin but also to function independently of both FADD and caspase 8 (Fig. 2B and 3). Thus, E8 and c-FLIPL seem to exhibit different effects on Wnt signaling. We suppose, however, that c-FLIPL might also possess the same Wnt signaling-enhancing activity as E8 downstream of the stabilization of β-catenin in various cells, in addition to the activity mimicking Wnt-induced signaling in 293T cells.
v-FLIP has been shown to be associated with tumorigenesis (11, 46), such as the development of Kaposi's sarcoma and primary effusion lymphoma, although its effect is regarded to be due to the activation of NF-κB and to be restricted to K13. It has previously been reported that E8 is involved in neither cell proliferation nor tumorigenesis (46), and it has been thought to function only as an inhibitor of apoptosis. However, because E8 was shown to induce the expression of endogenous cyclin D1 through enhancing Wnt3a signaling, it is likely that E8 can promote cell proliferation, and we found additional functions of E8 as a potent effector of cell proliferation. While Wnt3a signaling appears to slightly enhance and suppress the growth of KB and BALB/3T3 cells, respectively (see Fig. S3A and B in the supplemental material), E8 markedly enhanced the response of these cells to Wnt3a in a colony formation assay (Fig. 5). In the presence of E8, Wnt3a induces the growth stimulation of KB cells but not of U2OS and FL cells, and Wnt3a induces dramatic growth suppression or cell death in BALB/3T3 and MEF cells. To show that these effects were completely dependent on Wnt signaling, we analyzed the effect of ICAT. Although the expression of ICAT alone enhanced the growth of both KB and BALB/3T3 cells in the absence of E8 and Wnt3a (Fig. 5D and F), we were also able to show that ICAT definitely inhibits E8's regulation of the growth of KB and BALB/3T3 cells through Wnt3a signaling. E8 inhibits the proliferation of BALB/3T3 and MEF cells but does not show such an inhibitory effect on U2OS, KB, and FL cells. The different effects on cell proliferation between these cells may depend on different features of the cells; U2OS, KB, and FL cells are transformed, while BALB/3T3 and MEF cells are essentially untransformed. From this perspective, the phenotype of E8 Tg mice, which show a severe drop in thymocyte yield, might be explained by Wnt-induced growth retardation or death induction in thymocytes in the presence of E8. In conclusion, this work provides the basis of a novel in vitro system to analyze Wnt signaling in tumorigenesis, as well as the induction of differentiation or cell death. We are now performing further studies (i) to elucidate the molecular mechanisms of the E8-induced enhancement of Wnt signaling by identifying the target molecule that directly binds to E8 and (ii) to determine the basis of the dramatic Wnt3a-induced effects on cell growth in the presence of E8.
Supplementary Material
Acknowledgments
We are grateful to M. Hijikata, K. Shimotohno, K. Ueda, and J. Fujisawa for kindly providing research materials, and we thank H. Sakamaki, K. Lee, T. Shimaoka, and K. Okamoto for useful discussions.
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of the Japanese Government. S. Nakagiri was supported by the 21st Century COE Program of the MEXT, awarded to the Graduate School of Biostudies and Institute for Virus Research, Kyoto University.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene 22:3371-3385. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, S., C. N. Kim, W. C. Yeh, T. W. Mak, K. Bhalla, and G. N. Barber. 1998. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 17:6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrens, J., J. P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382:638-642. [DOI] [PubMed] [Google Scholar]
- 5.Belanger, C., A. Gravel, A. Tomoiu, M. E. Janelle, J. Gosselin, M. J. Tremblay, and L. Flamand. 2001. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 4:62-73. [PubMed] [Google Scholar]
- 6.Bertin, J., R. C. Armstrong, S. Ottilie, D. A. Martin, Y. Wang, S. Banks, G. H. Wang, T. G. Senkevich, E. S. Alnemri, B. Moss, M. J. Lenardo, K. J. Tomaselli, and J. I. Cohen. 1997. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldin, M. P., T. M. Goncharov, Y. V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 8.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene 18:5738-5746. [DOI] [PubMed] [Google Scholar]
- 10.Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505-512. [DOI] [PubMed] [Google Scholar]
- 11.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field, N., W. Low, M. Daniels, S. Howell, L. Daviet, C. Boshoff, and M. Collins. 2003. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 116:3721-3728. [DOI] [PubMed] [Google Scholar]
- 13.Goltsev, Y. V., A. V. Kovalenko, E. Arnold, E. E. Varfolomeev, V. M. Brodianskii, and D. Wallach. 1997. CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 272:19641-19644. [DOI] [PubMed] [Google Scholar]
- 14.Gounari, F., I. Aifantis, K. Khazaie, S. Hoeflinger, N. Harada, M. M. Taketo, and H. von Boehmer. 2001. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat. Immunol. 2:863-869. [DOI] [PubMed] [Google Scholar]
- 15.Guasparri, I., S. A. Keller, and E. Cesarman. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Han, D. K., P. M. Chaudhary, M. E. Wright, C. Friedman, B. J. Trask, R. T. Riedel, D. G. Baskin, S. M. Schwartz, and L. Hood. 1997. MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death. Proc. Natl. Acad. Sci. USA 94:11333-11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht, A., and M. P. Stemmler. 2003. Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. J. Biol. Chem. 278:3776-3785. [DOI] [PubMed] [Google Scholar]
- 18.Hu, S., C. Vincenz, M. Buller, and V. M. Dixit. 1997. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J. Biol. Chem. 272:9621-9624. [DOI] [PubMed] [Google Scholar]
- 19.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 20.Itoh, N., S. Yonehara, A. Ishii, M. Yonehara, S. Mizushima, M. Sameshima, A. Hase, Y. Seto, and S. Nagata. 1991. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233-243. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, N., S. Yonehara, J. Schreurs, D. M. Gorman, K. Maruyama, A. Ishii, I. Yahara, K. Arai, and A. Miyajima. 1990. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science 247:324-327. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka, T., R. C. Budd, N. Holler, M. Thome, F. Martinon, M. Irmler, K. Burns, M. Hahne, N. Kennedy, M. Kovacsovics, and J. Tschopp. 2000. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 10:640-648. [DOI] [PubMed] [Google Scholar]
- 23.Kazama, H., and S. Yonehara. 2000. Oncogenic K-Ras and basic fibroblast growth factor prevent Fas-mediated apoptosis in fibroblasts through activation of mitogen-activated protein kinase. J. Cell Biol. 148:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikuchi, A. 2000. Regulation of beta-catenin signaling in the Wnt pathway. Biochem. Biophys. Res. Commun. 268:243-248. [DOI] [PubMed] [Google Scholar]
- 25.Kolligs, F. T., G. Hu, C. V. Dang, and E. R. Fearon. 1999. Neoplastic transformation of RK3E by mutant β-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 19:5696-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 27.Lee, K. K., T. Ohyama, N. Yajima, S. Tsubuki, and S. Yonehara. 2001. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276:19276-19285. [DOI] [PubMed] [Google Scholar]
- 28.Liu, L., M. T. Eby, N. Rathore, S. K. Sinha, A. Kumar, and P. M. Chaudhary. 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J. Biol. Chem. 277:13745-13751. [DOI] [PubMed] [Google Scholar]
- 29.Logan, C. Y., and R. Nusse. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781-810. [DOI] [PubMed] [Google Scholar]
- 30.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 101:9399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molenaar, M., M. van de Wetering, M. Oosterwegel, J. Peterson-Maduro, S. Godsave, V. Korinek, J. Roose, O. Destree, and H. Clevers. 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391-399. [DOI] [PubMed] [Google Scholar]
- 33.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 34.Nagata, S. 1999. Fas ligand-induced apoptosis. Annu. Rev. Genet. 33:29-55. [DOI] [PubMed] [Google Scholar]
- 35.Naito, M., R. Katayama, T. Ishioka, A. Suga, K. Takubo, M. Nanjo, C. Hashimoto, M. Taira, S. Takada, R. Takada, M. Kitagawa, S.-I. Matsuzawa, J. C. Reed, and T. Tsuruo. 2004. Cellular FLIP inhibits β-catenin ubiquitylation and enhances Wnt signaling. Mol. Cell. Biol. 24:8418-8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.OhYama, T., S. Tsukumo, N. Yajima, K. Sakamaki, and S. Yonehara. 2000. Reduction of thymocyte numbers in transgenic mice expressing viral FLICE-inhibitory protein in a Fas-independent manner. Microbiol. Immunol. 44:289-297. [DOI] [PubMed] [Google Scholar]
- 37.Peifer, M., and P. Polakis. 2000. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606-1609. [DOI] [PubMed] [Google Scholar]
- 38.Prinos, P., S. Joseph, K. Oh, B. I. Meyer, P. Gruss, and D. Lohnes. 2001. Multiple pathways governing Cdx1 expression during murine development. Dev. Biol. 239:257-269. [DOI] [PubMed] [Google Scholar]
- 39.Sakamaki, K., T. Inoue, M. Asano, K. Sudo, H. Kazama, J. Sakagami, S. Sakata, M. Ozaki, S. Nakamura, S. Toyokuni, N. Osumi, Y. Iwakura, and S. Yonehara. 2002. Ex vivo whole-embryo culture of caspase-8-deficient embryos normalize their aberrant phenotypes in the developing neural tube and heart. Cell Death Differ. 9:1196-1206. [DOI] [PubMed] [Google Scholar]
- 40.Sekiya, T., S. Adachi, K. Kohu, T. Yamada, O. Higuchi, Y. Furukawa, Y. Nakamura, T. Nakamura, K. Tashiro, S. Kuhara, S. Ohwada, and T. Akiyama. 2004. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J. Biol. Chem. 279:6840-6846. [DOI] [PubMed] [Google Scholar]
- 41.Shibamoto, S., K. Higano, R. Takada, F. Ito, M. Takeichi, and S. Takada. 1998. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 3:659-670. [DOI] [PubMed] [Google Scholar]
- 42.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu, H. B., D. R. Halpin, and D. V. Goeddel. 1997. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity 6:751-763. [DOI] [PubMed] [Google Scholar]
- 44.Slusarski, D. C., J. Yang-Snyder, W. B. Busa, and R. T. Moon. 1997. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182:114-120. [DOI] [PubMed] [Google Scholar]
- 45.Staal, F. J., J. Meeldijk, P. Moerer, P. Jay, B. C. van de Weerdt, S. Vainio, G. P. Nolan, and H. Clevers. 2001. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur. J. Immunol. 31:285-293. [DOI] [PubMed] [Google Scholar]
- 46.Sun, Q., S. Zachariah, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-kappaB activation. J. Biol. Chem. 278:52437-52445. [DOI] [PubMed] [Google Scholar]
- 47.Tago, K., T. Nakamura, M. Nishita, J. Hyodo, S. Nagai, Y. Murata, S. Adachi, S. Ohwada, Y. Morishita, H. Shibuya, and T. Akiyama. 2000. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 14:1741-1749. [PMC free article] [PubMed] [Google Scholar]
- 48.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 49.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 50.Thome, M., and J. Tschopp. 2001. Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1:50-58. [DOI] [PubMed] [Google Scholar]
- 51.Tsukumo, S. I., and S. Yonehara. 1999. Requirement of cooperative functions of two repeated death effector domains in caspase-8 and in MC159 for induction and inhibition of apoptosis, respectively. Genes Cells 4:541-549. [DOI] [PubMed] [Google Scholar]
- 52.Ueda, Y., M. Hijikata, S. Takagi, R. Takada, S. Takada, T. Chiba, and K. Shimotohno. 2002. Wnt/beta-catenin signaling suppresses apoptosis in low serum medium and induces morphologic change in rodent fibroblasts. Int. J. Cancer 99:681-688. [DOI] [PubMed] [Google Scholar]
- 53.van de Wetering, M., R. Cavallo, D. Dooijes, M. van Beest, J. van Es, J. Loureiro, A. Ypma, D. Hursh, T. Jones, A. Bejsovec, M. Peifer, M. Mortin, and H. Clevers. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789-799. [DOI] [PubMed] [Google Scholar]
- 54.Wu, Z., M. Roberts, M. Porter, F. Walker, E. J. Wherry, J. Kelly, M. Gadina, E. M. Silva, G. A. DosReis, M. F. Lopes, J. O'Shea, W. J. Leonard, R. Ahmed, and R. M. Siegel. 2004. Viral FLIP impairs survival of activated T cells and generation of CD8+ T cell memory. J. Immunol. 172:6313-6323. [DOI] [PubMed] [Google Scholar]
- 55.Yanagawa, S., F. van Leeuwen, A. Wodarz, J. Klingensmith, and R. Nusse. 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9:1087-1097. [DOI] [PubMed] [Google Scholar]
- 56.Yeh, W. C., J. L. Pompa, M. E. McCurrach, H. B. Shu, A. J. Elia, A. Shahinian, M. Ng, A. Wakeham, W. Khoo, K. Mitchell, W. S. El-Deiry, S. W. Lowe, D. V. Goeddel, and T. W. Mak. 1998. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279:1954-1958. [DOI] [PubMed] [Google Scholar]
- 57.Yonehara, S., A. Ishii, and M. Yonehara. 1989. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J. Exp. Med. 169:1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yonehara, S., A. Ishii, M. Yonehara, S. Koyasu, A. Miyajima, J. Schreurs, K. Arai, and I. Yahara. 1990. Identification of a cell surface 105 kd protein (Aic-2 antigen) which binds interleukin-3. Int. Immunol. 2:143-150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.