Abstract
During embryogenesis, various cell types can be programmed by potent inducers to follow distinct differentiation paths. In adult life, this ability seems to be restricted to specific multipotent cells. We have identified two cell populations from adult murine bone marrow which express various “stemness” genes. Treatment with Wnt molecules induced transcription of different skeletal muscle marker genes and evoked expression of cardiomyocyte markers. Further characterization of Wnt-induced intracellular signaling cascades revealed that the skeletal muscle program depended on canonical Wnt signaling, while the induction of cardiomyocyte markers seems to require a protein kinase C-dependent pathway. CDO, another component of the machinery directing skeletal muscle induction and expansion, selectively activated skeletal muscle- but not cardiomyocyte-specific genes. Although we were able to turn on various cell-type-specific markers by different induction regimens, we never obtained fully differentiated, functional cells. We conclude that the differentiation of adult stem cells is incomplete and lacks certain cues necessary to acquire a truly functional status.
Tissue-specific stem cells contribute to the regeneration and maintenance of numerous if not all tissues of mammals, including blood, liver, intestine, skeletal muscle, and the central nervous system. It is generally believed that tissue-specific stem cells are determined to follow specific cellular fates and contribute only to the tissue from which they originate. This paradigm has been challenged recently. Several studies postulated the presence of adult stem cells capable of differentiating into a broad spectrum of specialized cells. For instance, it has been claimed that adult neuronal stem cells isolated from the brain tissue of the mouse differentiate into blood, skeletal muscle, and endothelial cells (4, 28, 36), that cells from human adipose tissue differentiate into bone, muscle, and cartilage (39), and that cells from the dermis of mammalian skin differentiate into skeletal muscle, neuron, glial, and fat cells (34). Furthermore, several studies proposed that bone marrow-derived cells have the capacity to differentiate not only into blood but also into various other cell types, such as muscle (11, 13), brain (6), and liver (33), among others. It has also been claimed that a single rare cell population, so-called multipotent adult progenitor cells, is able to differentiate into derivatives of all three germ layers (17). Since such cells can be isolated and handled with relative ease, they have been considered attractive vehicles for somatic gene and cellular therapy.
Although the significance and validity of some of these studies have been questioned, it seems clear that certain stromal cells of the bone marrow (and probably also of other organs) can respond to various stimuli in vitro and in vivo (26) by expression of cell-type-specific marker molecules. Distinct cell types within the rather diverse stromal cell population can be defined by their expressions of specific surface marker molecules, their levels of adherence to various substrates, the ability for self-renewal, and their responses to various stimuli (17).
Unfortunately, several of the parameters, which are of critical importance for the isolation and characterization of distinct stromal cell populations, might vary from one lab to another. Since the stroma consists of various different mesenchymal cell types, it is usually necessary to separate distinct cell populations based on fluorescence-activated cell sorting (FACS) and/or the adherence properties of these cells to cell culture dishes, subsequent culture conditions, and other treatments (17). Obviously, such procedures might lead to the isolation and growth of slightly different cell types with different properties in various assays. In addition, cocultures of different cell types and transplantation of cells into host animals are prone to all types of labeling and detection artifacts. At present, it is not clear whether bone marrow-derived cells or other circulating cells play any significant role that can be attributed to the incorporation of these cells into diseased tissues. Alternatively, it seems possible that some of the beneficial effects observed after the infusion of stem cells rely on the induction of proliferation of resident cells by grafted cells.
A paradigmatic example for the ongoing debate about the specific functions of different cell types in tissue regeneration is the skeletal muscle. Despite a wide agreement that muscle satellite cells represent the main source of muscle stem cells, other cell populations which either reside within skeletal muscles or are derived from the bone marrow have been proposed to contribute to muscle regeneration. These cell types, which have been collectively named adult stem cells, include the so-called side population (13, 16) and CD45+ cells resident in skeletal muscle (25). While satellite cells readily give rise to differentiated myocytes in culture, adult stem cells behave differently. It has been claimed that side population cells differentiate spontaneously at a low degree into myotubes and hematopoietic cells (2), while C45+ cells depend on Wnt signaling to initiate the myogenic differentiation cascade (25). On the other hand, Zhao and Hoffman (37) reported that none of the Wnt members discussed by Polesskaya et al. (25) are active in vivo, raising doubts whether Wnt signaling significantly contributes to the regeneration of adult muscle tissue in vivo by recruiting nonmuscle cells residing within the muscle tissue.
Here, we have focused on the plasticity of murine bone marrow-derived cells and on signals that are known to be involved in the control of myogenesis, namely, Wnts and CDO, which direct skeletal muscle cell lineage induction and expansion during embryogenesis. We examined the effects of Wnts and CDO on two different murine bone marrow-derived multipotent adult stem cell populations (mBM-MASCs) which we isolated from whole bone marrow and named mBM-MASCs1 (CD34−/Sca-1high) and mBM-MASCs2 (CD34+/Sca-1moderate). In addition, we analyzed the inherent multilineage potentials of these cells after epigenetic reprogramming and treatment with different growth factor combinations. Although we were able to induce the expression of molecular markers characteristic of all three germ layers in a subset of treated cells, we did not obtain fully functional cells. Our results suggest that the differentiation of mBM-MASCs depends on stochastic events and is arrested prior to terminal differentiation, probably due to the absence of critical determination events.
MATERIALS AND METHODS
Isolation and cultivation of mBM-MASCs.
mBM-MASCs were isolated from the bone marrow of 2-month-old female ICR mice by expansion of individual clones derived from rare, slowly dividing cells. Clones were recovered and subjected to additional rounds of plating and growth until homogenous cell populations were obtained. Details of the procedure are described in the supplemental material. Two cell populations which showed differential expression of a selected cell surface marker, designated mBM-MASCs1 and mBM-MASCs2, were characterized further.
Flow cytometry.
mBM-MASCs were characterized at various time points by standard flow cytometry using antibodies against Sca-1, c-Kit, CD34, CD45, Ter119, CD13, SSEA-1 (stage-specific embryonic antigen), CD133/prominin, Flk-1, and H-2Dd. Data collected from >10,000 cells were expressed as the percentage of positive cells from the total gated cell population. Raw data were analyzed using the CellQuest Pro software (BD Inc.).
RNA isolation and RT-PCR.
Total RNA was isolated from adult mouse skeletal muscle, heart, liver, and brain tissues and differentiated and undifferentiated mBM-MASCs with Trizol (Invitrogen). Reverse transcription-PCR (RT-PCR) analyses were performed using 1 μg of DNase-treated RNA isolated from various tissues and differentiated and undifferentiated mBM-MASCs as described previously (21). Detailed protocols and primer sequences are available from the authors on request. In all cases, a housekeeping gene, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, was used as an internal control. Identities of PCR products were corroborated by DNA sequence analysis and hybridization with radioactively labeled probes.
Construction of recombinant retroviruses.
Mouse expressed sequence tag clones containing the complete coding regions of CDO (cell adhesion molecule down-regulated by oncogene) IMAGp998C168558Q3 and Wnt11 (Wingless-related mouse mammary tumor virus integration site 11) IMAGp998G23800 were obtained from Deutsches Ressourcenzentrum für Genomforschung, GmbH. Respective cDNA fragments were released with appropriate restriction enzymes and inserted into pMSCVneo (Clontech). All constructs were verified by sequence analysis. Recombinant viral particles were generated by transfection of either pMSCVneo-Wnt11 or pMSCVneo-CDO together with the gag-pol expression constructs pM57 and M108 into the retroviral packaging cell lines C2BAC and Phoenix (ATCC) as described previously (23). The myogenin promoter-enhanced green fluorescent protein (eGFP) lentiviral reporter virus was a kind gift of D. Baltimore (Caltech, Pasadena, Calif.) (20).
Generation of Wnt- and CDO-expressing cells.
Retroviral constructs encoding Wnt7A, Wnt7B, and Wnt4 were generously provided by Jan Kitajewski (Columbia University New York, N.Y.) and used to transfect Psi2 packaging cells using standard procedures (23). Stably transfected clones were isolated after G418 treatment and used to generate high-titer virus preparations. For coculture experiments, we used either packaging cells expressing different Wnts and CDO or NIH 3T3 cells which had been infected with different recombinant retroviruses. Infected cells were treated with mitomycin C (GIBCO BRL) to inhibit cell proliferation and to prevent the overgrowth of inducing cells.
Induction of cell differentiation.
For coculture experiments, mBM-MASCs1 and -2 were seeded in six-well tissue culture plates (Nunc) at 1 × 105 cells/well. After 24 h, the same number of amitotic feeder cells expressing Wnt signaling molecules or CDO was added. Cultures were maintained in low-glucose Dulbecco's modified Eagle's medium supplemented with 3% (vol/vol) fetal calf serum for 7 or 8 days, with a medium change at day 4. To initiate epigenetic reprogramming, mBM-MASCs1 and -2 were seeded in six-well tissue culture plates (Nunc) at 1 × 105 cells/well. The next day, different concentrations of 5-azacytidine (AZA) (5, 10, and 15 μmol/liter), trichostatin A (TSA; 0.1, 0.3, and 0.9 μmol/liter), or a combination of both 5-AZA and TSA (5 and 0.1, 10 and 0.3, and 15 and 0.9 μmol/liter) (obtained from Sigma) were added. After 24 h, the medium was changed and cells were maintained in low-glucose Dulbecco's modified Eagle's medium supplemented with 3% fetal calf serum (vol/vol) for 10 days with a single medium change at day 4. In some experiments, 5-azacytidine was added as indicated above, but cells were maintained in culture for either 15 or 21 days. In order to explore the effects of various growth factors, cells seeded in six-well tissue culture plates (Nunc) at 1 × 105 cells/well were treated with different concentrations of fibroblast growth factor 2, bone morphogenetic protein 2, hepatocyte growth factor/scatter factor, or combinations of various growth factors as indicated below. Inhibition of protein kinase C (PKC) activity was achieved by treatment of mBM-MASCs with different concentrations of staurosporine and bisindolylmaleimide I as indicated. After 12 h, cells expressing the Wnt11 molecule were added, and the cultures were incubated for an additional 7 to 8 days. Medium including the inhibitors was changed every 3 days.
Immunofluorescence staining.
Sarcomeric myosin heavy chain (MHC) was detected with the MF-20 monoclonal antibody isolated from the supernatant of hybridoma cells as described previously (5). Monoclonal antibodies against cardiac troponin T (anti-cTnT; 1:200) and troponin I (anti-cTnI; 1:200) were purchased from DPC Biermann. The use of the myogenin antibody has been described before (12). Staining was accomplished with secondary antibodies coupled with Alexa 488 or Alexa 594 (Chemicon). Nuclear staining was performed using Hoechst 33258 (Dako) at 5 μg/ml for 10 min.
RESULTS
Isolation of two multipotent cell populations (mBM-MASCs) from adult murine bone marrow.
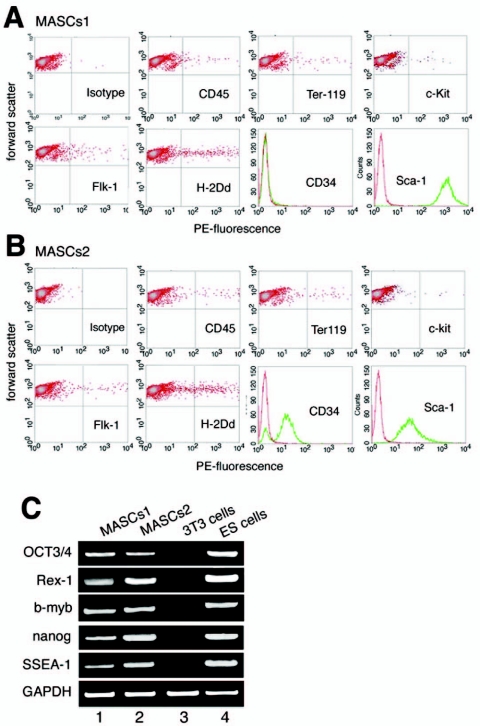
Initial attempts to activate myogenic pathways in nonpurified populations of mesenchymal cells derived from the bone marrow proved to be highly variable and biased by occasional contaminations with myoblasts and yielded no conclusive results in our hands (see Fig. S1A in the supplemental material), although it has been claimed by other groups that full myogenic differentiation might be accomplished using unselected bone marrow-derived mesenchymal cells (29). To achieve a reproducible outcome in a defined experimental system, we decided to use individual cell populations which express distinct sets of marker genes. We therefore isolated two separate multipotent mesenchymal cell populations from the bone marrow as described in Materials and Methods. Morphologically, cells from both populations displayed large nuclei and scanty cytoplasms and exhibited roughly the same doubling times. Initially, cultures went through phases of dormancy of nearly a month before homogenous mBM-MASCs were recovered by a series of sequential passages (see Fig. S1B in the supplemental material). FACS analysis (forward and sideward scatter) indicated that the cell population consisted mostly of large and moderately granular cells, with a minor portion of small cells. FACS-assisted immunocytometry for expression of Sca-1, c-Kit, CD34, CD45, Ter119, Flk-1, and H-2Dd (Fig. 1) as well as CD13, SSEA-1, and CD133/prominin (data not shown) revealed a clear difference in the expression levels of CD34 and different expression levels of Sca-1 in the two cell populations, while other parameters were virtually identical. In summary, mBM-MASCs1 were CD34− Sca-1high CD45dim Ter119dim Flk-1low H-2Dd low, and mBM-MASCs2 were CD34+ Sca-1moderate CD45dim Ter119dim Flk-1low H-2Dd low. A dot plot representation of the expressions of c-Kit, CD45, Ter119, Flk-1, and H-2Dd indicated some heterogeneity in the population, with few cells expressing the antigens at high concentrations and a large bulk of cells which showed only minor or no expression. (Fig. 1). CD45, Ter119, c-Kit, and Flk-1 were found in less than 0.5% and H-2Dd in no more than 2% of all cells. Interestingly, this ratio remained constant even after extended subculturing, suggesting a stochastic process that leads to the occasional activation of distinct sets of permissive genes.
FIG. 1.
Analysis of stem cell marker gene expression in mBM-MASCs. (A) FACS analysis of uninduced mBM-MASCs1 and -2. Expression of Sca-1, c-Kit, CD34, CD45, Ter119, Flk-1, and H-2Dd surface markers was analyzed by staining with phycoerythrin (PE)-conjugated monoclonal antibodies. mBM-MASCs1 and -2 differed in their expressions of Sca-1 and CD34. No expression of CD34 was found in mBM-MASCs1. Negative peaks in the CD34 and Sca-1 graphs represent phycoerythrin-conjugated isotype controls. (B) RT-PCR analysis of the expression of alleged stemness genes in mBM-MASCs1 and -2, embryonic stem (ES) cells, and NIH 3T3 cells. mBM-MASCs1 and -2 showed robust expressions of OCT3/4, Rex-1, b-myb, nanog, and SSEA-1 (stage-specific embryonic antigen) (lanes 1 and 2). Embryonic stem cells (lane 4) were used a positive control, and NIH 3T3 cells (lane 3) were used as a negative control.
Various Wnt signaling molecules share similar potentials to activate the myogenic program in mBM-MASCs.
Wnt molecules are well-known inducers of myogenesis during embryonic development. We wanted to explore whether myogenic differentiation might be induced in mBM-MASCs by various Wnt molecules and whether differences in the inductive potentials of different Wnt molecules in this assay system might exist.
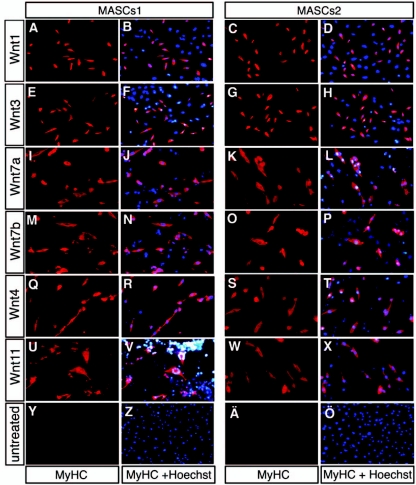
We therefore cocultured the two different mBM-MASC populations together with amitotic feeder cell lines that secreted Wnt1, Wnt3, Wnt7a, Wnt7b, Wnt4, or Wnt11. After 7 days, we noted profound morphological changes in mBM-MASCs1 (CD34− Sca-1high). Cells switched from a fibroblastlike phenotype to an appearance that resembled that of small, mononucleated primary myotubes. mBM-MASCs2 (CD34+ Sca-1moderate) reacted similarly, although it took these cells 1 to 2 days longer to acquire the same differentiation-related morphological changes. Treatment of mBM-MASCs with Wnt molecules also resulted in a clear reduction of the proliferation rate compared to that for nontreated cells (data not shown). We next stained Wnt-treated mBM-MASCs by immunofluorescence with an antibody (MF-20) that detects sarcomeric MHC and with a monoclonal antibody directed against myogenin (data not shown). As shown in Fig. 2, approximately 10% of all mBM-MASCs stained positive for MHC, no matter which cell population and Wnt signaling molecule were used (Fig. 2A through X). We did not observe the formation of fused multinucleated myotubes that is characteristic of the differentiation of bona fide myoblasts, suggesting that we were unable to achieve a complete myogenic programming of mBM-MASCs. Changes in culture conditions, including additions of insulin-like growth factor 1, dexamethasone, insulin, and epidermal growth factor, either alone or in combination, had no effect on this outcome (data not shown). No MHC staining was observed in untreated mBM-MASC controls (Fig. 2Y through Ö). To rule out the (unlikely) possibility that fusion of Wnt-expressing cells to mBM-MASCs is required for the initiation of the myogenic program, we repeated the induction experiments by placing Wnt-secreting cells on one side of a membrane with defined pore sizes and the responding mBM-MASCs on the other side. Initiation of the myogenic program, as indicated by MHC expression, was evident even when 0.4-μm-pore-size membranes, which prevent the transmigration of cells through the membrane, were used (see Fig. S1C in the supplemental material).
FIG. 2.
Different Wnt signaling molecules induce expression of MHC in mBM-MASCs. mBM-MASCs1 and -2 were cocultured with amitotic cells that expressed either Wnt1 (A to D), Wnt3 (E to H), Wnt7a (I to L), Wnt7b (M to P), Wnt4 (Q to T), or Wnt11 (U to X) or with the parental cell line (Y to Ö). After 7 to 8 days, cultures were stained with the monoclonal MF-20 antibody against sarcomeric myosin heavy chain and a secondary antibody coupled to Alexa 594 anti-mouse. MHC expression was indiscriminately activated by all Wnt molecules tested. Nuclei were counterstained with Hoechst 33258 to locate all cells on the plate (B, F, J, N, R, V, and Z [second column] and D, H, L, P T, X, and Ö [fourth column]). MyHC, MHC.
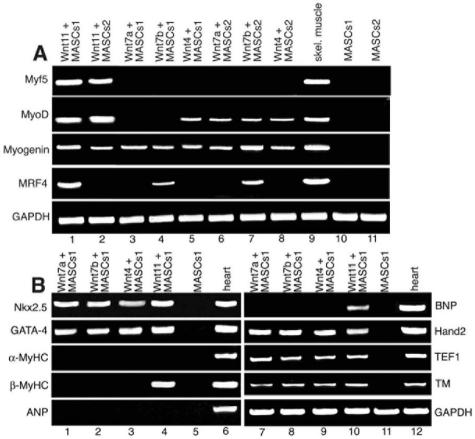
To further analyze the initiation of the skeletal muscle program in mBM-MASCs, we examined the expression of the four skeletal muscle-specific myogenic determination factors, i.e., Myf5, MyoD, myogenin, and myogenic regulatory factor 4 (MRF4), by RT-PCR 8 days after the initiation of Wnt treatment. As shown in Fig. 3, we always found expression of at least one myogenic factor after induction with different Wnt molecules, although some differences between the effects of individual Wnt molecules were obvious. Induction of mBM-MASCs1 with Wnt11 resulted in expression of all four myogenic factors, while Wnt7a stimulated only the expression of myogenin after continuous treatment. Under our conditions, the expression of Myf5 was activated solely by Wnt11, while the expression of MRF4 depended on Wnt11 and Wnt7b. Even though no systematic study of the temporal expression patterns of MRFs during treatment of mBM-MASCs with different Wnt molecules was performed, changes in the expression profiles of MRFs were observed. Expression of MyoD, for example, was present in most cultures of mBM-MASCs early after the initiation of Wnt treatment but faded at later stages, although this finding varied considerably between individual experiments (data not shown).
FIG. 3.
Induction of skeletal and cardiac muscle marker genes in mBM-MASCs by different Wnt molecules. (A) RT-PCR analysis of the expression of skeletal muscle myogenic factors. RNA was isolated from mBM-MASCs1 and -2, which were cocultured for 7 days with different amitotic Wnt-expressing cells (lanes 1 to 8), with the parental cell line lacking Wnt expression (lanes 10 and 11), and with skeletal (skel.) muscle cells (lane 9). Different expression patterns of myogenic factors were induced. Treatment with Wnt molecules resulted in the activation of at least one myogenic factor. (B) RT-PCR analysis of the expression of cardiac muscle marker genes using the same experimental setup. Wnt11 was the only Wnt molecule that activated expression of β-MHC and BNP (lanes 4 and 10), while other Wnt molecules stimulated Nkx-2.5, Hand2, and GATA-4 expression. All Wnt molecules failed to induce α-MHC and ANP. MyHC, MHC.
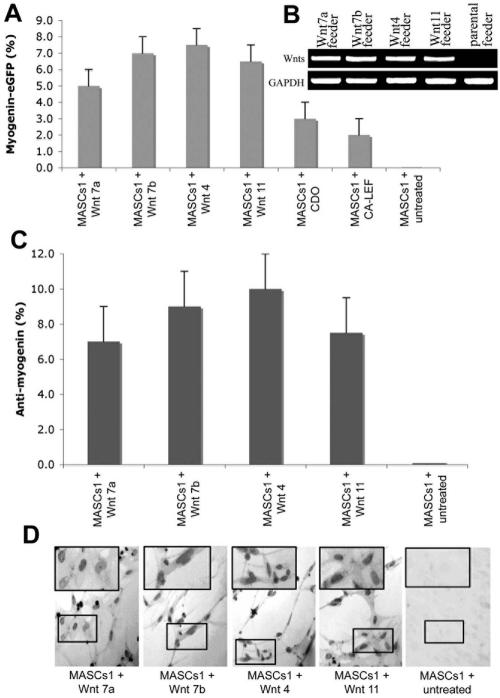
We next assessed the number of myogenin-positive cells after treatment with different Wnt molecules by using myogenin antibody staining or FACS analysis of mBM-MASCs infected with a myogenin promoter-eGFP retroviral reporter construct (Fig. 4A). Expression of myogenin protein was found in up to 10% of all cells, which roughly matched the number of MHC-positive cells (Fig. 4C and D). The myogenin promoter-eGFP reporter, which allows analysis of much larger cell numbers, was activated to a similar degree (Fig. 4A). Despite the fact that all Wnts were expressed at comparable levels in inducing feeder cells, as assessed by semiquantitative RT-PCR (Fig. 4B), we noted consistent differences in the efficiencies of Wnts in activating myogenin expression. However, at present it is hard to decide whether this is due to intrinsic biochemical differences between Wnt molecules or different bioavailabilities of Wnt proteins.
FIG. 4.
Comparison of the numbers of myogenin-expressing mBM-MASCs after induction with different Wnts, CDO, and CA-LEF. (A) FACS-assisted cell counts of GFP-positive mBM-MASCs that had been stably infected with a myogenin promoter-eGFP retroviral reporter virus before treatment with various effectors. (B) Semiquantitative RT-PCR of the expression of different Wnt molecules in inducing feeder cells. (C) Number of myogenin-positive cells after staining with an anti-myogenin antibody. Numbers in panels A and C represent the percentages of positive cells within the whole cell population. Error bars indicate the standard deviations. (D) mBM-MASCs were induced with different Wnt-expressing cells and stained with an anti-myogenin antibody to reveal the presence of the nuclear antigen. Representative examples are shown.
Differential activation of cardiomyocyte markers by different Wnt molecules.
To investigate the potential of various Wnt molecules to activate the cardiac program in mBM-MASCs, we analyzed the expression of different cardiomyogenic marker genes by RT-PCR after induction with different Wnt molecules. As shown in Fig. 3, all Wnt molecules tested (Wnt4, Wnt7a, Wnt7b, Wnt11) induced expression of the Nkx-2.5, GATA-4, Hand 2 (dHand), TEF1, and tropomyosin cardiac marker genes, while only treatment with Wnt11 resulted in the expression of β-MHC and brain natriuretic protein (BNP) (Fig. 3B). However, we were unable to identify reproducible expression of other typical cardiomyocyte genes, such as the α-MHC and atrial natriuretic protein (ANP) genes (Fig. 3B). Similarly, the induction of mBM-MASCs by Wnt11 resulted in the detection of cTnT by immunofluorescence (Fig. 5D), but no organized contractile apparatus or cross-striations were discernible (Fig. 5D). Double immunofluorescence staining of Wnt11-induced mBM-MASCs using antibodies directed against GATA-4 and Nkx-2.5 revealed a high degree of heterogeneity among induced cells. Some cells expressed either GATA-4 or Nkx-2.5, some expressed both antigens, and some expressed neither of them (see Fig. S1D in the supplemental material). No cells that underwent spontaneous contractions were identified. We concluded that treatment of mBM-MASCs with Wnt molecules led only to the activation of certain cardiac marker genes and not to the full differentiation that would be necessary to acquire a truly functional status.
FIG. 5.
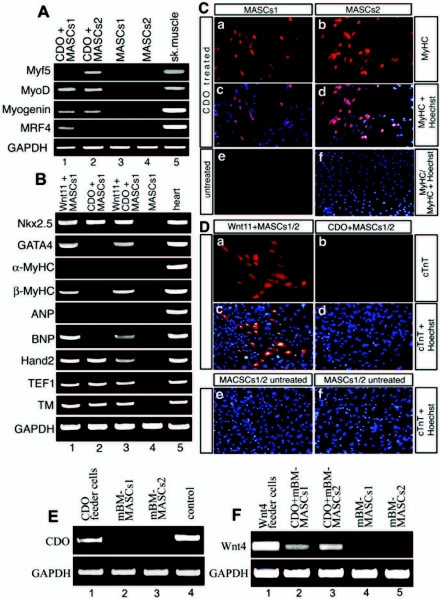
CDO induces the expression of skeletal muscle but not of cardiac muscle marker genes in mBM-MASCs. (A) RT-PCR analysis of the expression of skeletal muscle myogenic factors. RNA was isolated from mBM-MASCs1 and -2 that were cocultured for 7 days with amitotic CDO-expressing cells (lanes 1 and 2), with the parental cell line lacking CDO expression (lanes 3 and 4), and with skeletal muscle cells (lane 5). CDO treatment led to the robust expression of myogenic regulatory factors. (B) RT-PCR analysis of the expression of cardiac muscle marker genes using the same experimental setup. CDO (lane 2) failed to induce cardiac markers such as GATA4 and β-MHC, while Wnt11 alone (lane 1) or in combination with CDO (lane 4) had this ability. (C) Immunofluorescent staining of MASCs for MHC expression after cocultivation with CDO-expressing cells and with the parental cell line lacking CDO expression. Nuclei were counterstained with Hoechst 33258 (c, d, and f) to locate all cells on the plate. (D) Immunofluorescent staining of MASCs for cTnT expression after cocultivation with Wnt11- and CDO-expressing cells and with the parental cell line lacking CDO or Wnt11 expression. Nuclei were counterstained with Hoechst 3444 (c to f) to locate all cells on the plate. (E) RT-PCR analysis of CDO expression in feeder cells and MASCs. (F) RT-PCR analysis of Wnt4 expression in MASCs after induction with CDO. Note the induction of Wnt4 expression after CDO treatment. MyHC, MHC.
CDO activates skeletal muscle but not cardiac muscle markers in mBM-MASCs.
CDO is a ROBO-related cell surface protein that has been proposed to mediate effects of cell-cell interactions between muscle precursor cells and to participate in a positive feedback loop with MyoD to enhance skeletal myogenesis (19). To investigate whether directed expression of CDO might enhance the intrinsic propensity of mBM-MASCs, which express CDO at very low levels (Fig. 5E), to initiate expression of myogenic genes during stem cell differentiation, we cocultured mBM-MASCs1 and mBM-MASCs2 together with mitotically inactive feeder cells that carried high levels of the CDO molecule. As shown in Fig. 5, both mBM-MASCs1 and mBM-MASCs2 expressed MyoD and myogenin after 7 to 8 days of cocultivation, whereas MRF4 was exclusively expressed in mBM-MASCs1 and Myf5 in mBM-MASCs2 (Fig. 5A). In addition, we detected the expression of Wnt4 (Fig. 5F) but not of other Wnt molecules (data not shown) in CDO-stimulated MASCs, further supporting the hypothesis that CDO might enhance positive feedback loops to stimulate differentiation. The induction of mBM-MASCs by CDO also resulted in the expression of some sarcomeric proteins, as demonstrated by immunofluorescence detection of MHC (Fig. 5C). Despite a robust expression of MHC, we again did not detect multinucleated myotubes or organized sarcomeric structures, a result comparable to the observations made with Wnt-primed mBM-MASCs. Similar results were obtained using human BM-MASCs that were cocultured either with Wnt11- or CDO-expressing cells (see Fig. S1F in the supplemental material). Coculturing with CDO-expressing cells also resulted in a reduction of cell numbers in comparison to untreated plates seeded at the same initial density, most likely indicating cell cycle withdrawal as a consequence of (partial) cellular differentiation. To rule out the option that the initiation of the myogenic program by CDO depended on the fusion of CDO-expressing cells to mBM-MASCs, we performed the same filter experiment described above for Wnt-expressing cells. Similarly, we observed an induction of the myogenic program, as indicated by MHC expression, even when 0.4-μm-pore-size membranes, which prevent the transmigration of cells through the membrane, were used (see Fig. S1E in the supplemental material).
Although no role for CDO in the activation of cardiac muscle cell differentiation has been described so far, the ability of CDO to activate genes characteristic for striated muscle prompted us to analyze whether CDO-mediated cellular signaling processes might also cause the activation of cardiac muscle marker genes in mBM-MASCs. As shown in Fig. 5, CDO treatment led to the activation of the Nkx-2.5 gene but not to the expression of the GATA-4, α-MHC, β-MHC, ANP, or BNP genes. In this respect, the gene expression pattern evoked by CDO resembled that of Wnt4 and Wnt7, with the notable exception of a missing GATA-4 gene expression. Similarly, CDO-induced mBM-MASCs also failed to show cTnT protein expression (Fig. 5D), while Wnt11 (Fig. 5D) but not Wnt4 and Wnt7 (data not shown) clearly resulted in an induction of cTnT in a subset of stimulated cells. Combined application of CDO and Wnt11 did not prevent the activation of cardiac muscle-specific Wnt11 target genes, indicating that CDO does not affect cardiomyogenic pathways in a negative manner (Fig. 5B).
Activation of cardiomyocyte marker gene expression by Wnt molecules in mBM-MASCs depends on PKC but not on LEF signaling.
Wnt molecules are known to play a pivotal role in the induction of myogenesis during somitic development. Ectopic expression of Wnt-1 completely represses ventral (sclerotomal) markers and enhances and expands expression of dorsal (myogenic) markers in somites. Similarly, the delivery of an activated form of β-catenin to somitic mesoderm mimics the effects of Wnt-1, suggesting that Wnt signaling is mediated via the so-called canonical Wnt/β-catenin pathway (7). We wanted to explore whether the activation of skeletal and cardiac muscle genes in mBM-MASCs also depends on the canonical Wnt/β-catenin pathway. We therefore expressed a constitutively active form of LEF (CA-LEF) (1), which is an essential part of the β-catenin nuclear complex, by retrovirus-mediated gene transfer in mBM-MASCs and assayed the expression of tissue-specific marker genes. Directed expression of CA-LEF resulted in the activation of several skeletal muscle-specific genes, including the Pax7, Myf5, MyoD, and myogenin genes in mBM-MASCs (Fig. 6A). Likewise, we observed the activation of a myogenin promoter-GFP lentiviral reporter construct in a subset of mBM-MASCs (Fig. 6C). In contrast, we found an absence of cardiomyocyte markers in mBM-MASCs after CA-LEF expression, with the exception of Nkx-2.5 (Fig. 6B), which was also expressed after treatment with Wnt4, Wnt7a, and Wnt7b (Fig. 3B). These results clearly indicate that Wnt-mediated activation of cardiac marker genes in mBM-MASCs did not depend on the canonical Wnt/β-catenin pathway but was relayed by an alternative route. Although the precise mechanisms of noncanonical Wnt signaling have not been unveiled in detail, it seems clear that PKC plays an important role both in the Wnt/Jun N-terminal protein kinase and Wnt/Ca2+ pathways (24). We therefore treated mBM-MASCs that were cocultured with Wnt11-expressing cells with different concentrations of staurosporine and bisindolylmaleimide I, two known inhibitors of PKC. As shown in Fig. 7, the expression of Nkx2.5, myocardin, BNP, and β-MHC was completely abrogated after inhibition of PKC, while the expression of TEF-1, tropomyosin, and GAPDH remained unchanged. Surprisingly, the expression of GATA-4 was not affected by staurosporine and bisindolylmaleimide I. We even noted a slight increase in GATA-4 gene transcripts after PKC inhibition (Fig. 7). Taken together, our results suggest the activation of different sets of cardiac muscle-specific genes by multiple Wnt signaling branches.
FIG. 6.
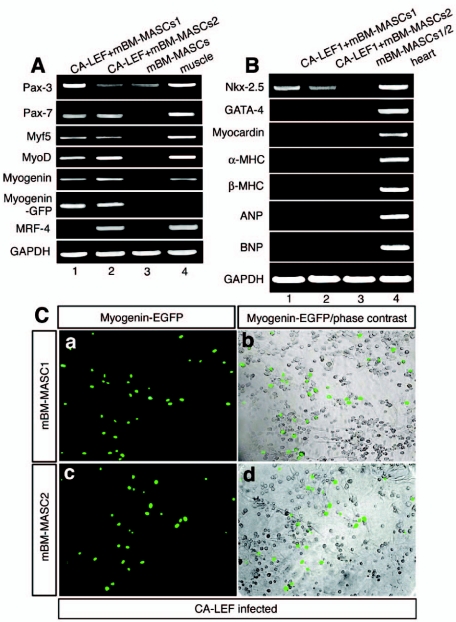
CA-LEF1 induces the expression of skeletal muscle but not of cardiac muscle marker genes in mBM-MASCs. RT-PCR analysis of the expression of skeletal muscle myogenic factor (A) and cardiac marker genes (B) after infection of mBM-MASCs1 and -2 with a retrovirus encoding CA-LEF. CA-LEF1 led to the activation of several skeletal muscle markers but not of cardiac marker genes, with the exception of the Nkx-2.5 gene. (C) mBM-MASCs1 and -2, each containing a myogenin promoter-eGFP reporter construct, were infected with a retrovirus encoding CA-LEF. Activation of the myogenin-eGFP reporter construct is shown under fluorescent light (a and c) and merged with corresponding phase-contrast images (b and d), indicating the activation of skeletal muscle genes.
FIG. 7.
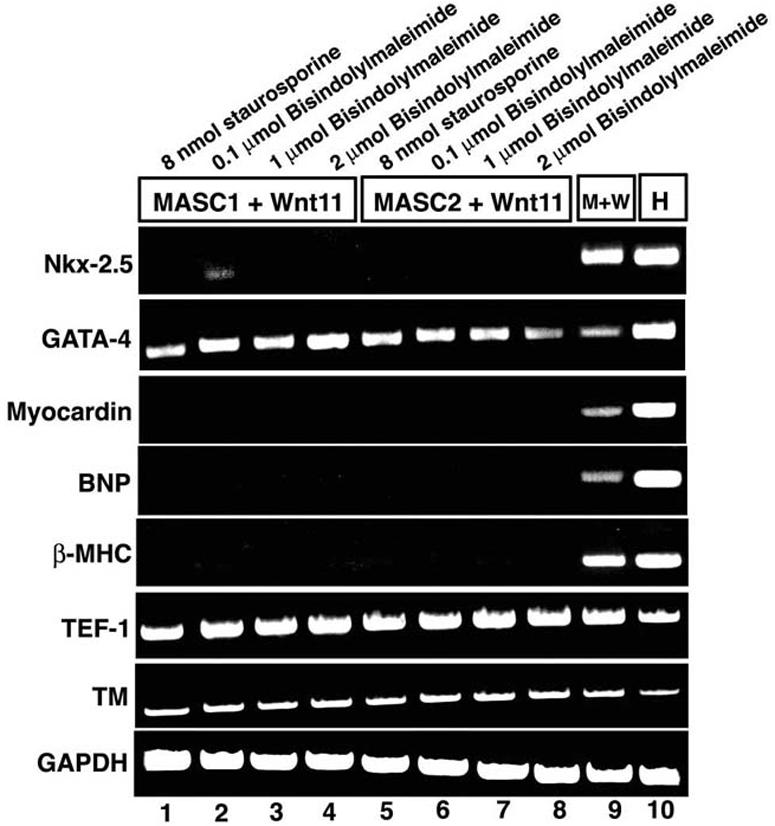
Inhibition of PKC abrogates activation of most cardiac markers by Wnt11 in mBM-MASCs. RT-PCR analysis of the expression of cardiac markers after coculturing of Wnt11-expressing cells with mBM-MASCs and treatment with various concentrations of bisindolylmaleimide I and staurosporine (lanes 1 to 8). RNAs isolated from cocultures without the addition of inhibitors and from heart tissue were used as controls. Expression of cardiac marker genes, with the exception of the GATA-4 gene, was efficiently repressed by the inhibitors.
Induction of chromatin remodelling leads to spontaneous expression of skeletal and cardiac muscle marker genes.
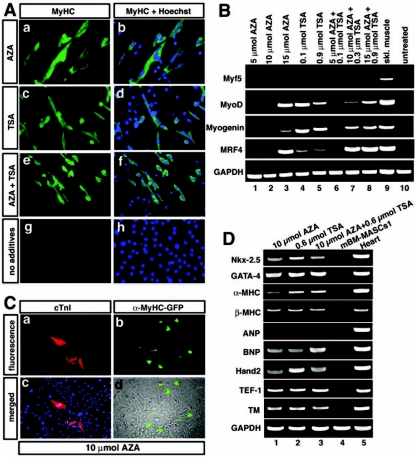
From our previous experiments, it became clear that mBM-MASCs own a considerable plasticity that allows them to activate parts of the skeletal and cardiac muscle cell programs in response to specific cues. Although specific signals are apparently required to efficiently turn on components of defined differentiation networks, a certain propensity for spontaneous determination events seemed inherent to mBM-MASCs. We wanted to investigate whether this plasticity might be enhanced by stimulation of chromatin remodelling and alleviation of gene silencing. We therefore incubated mBM-MASCs with AZA (an inhibitor of DNA methylation), TSA (an inhibitor of histone deacetylases), or a combination of both drugs and analyzed the expression of skeletal and cardiac muscle-specific genes. Treatment of mBM-MASCs with either drug (Fig. 8) resulted in activation of the myogenic factors MyoD, myogenin, and MRF4 (Fig. 8B) and of sarcomeric MHC (Fig. 8A). In contrast to the induction of the myogenic program by Wnts, we did not notice expression of Myf5 even after combined treatment with AZA and TSA. After treatment with AZA and TSA, we detected the expression of several cardiac marker genes, including the cTnI (Fig. 8C), GATA-4, Hand-2, and β-MHC (Fig. 8D) genes, in mBM-MASCs. Interestingly, we also found the expression of α-MHC, which was not present after induction of MASCs with Wnt11, and the activation of a transgenic eGFP reporter gene driven by the α-MHC promoter that had been stably introduced into mBM-MASCs (Fig. 8C). However, we failed to score the expression of ANP, indicating a partial recapitulation of the cardiac program despite the activation of the α-MHC gene.
FIG. 8.
Epigenetic reprogramming leads to enhanced plasticity and spontaneous differentiation of MASCs. (A) mBM-MASCs2 were stained with the monoclonal MF-20 antibody against sarcomeric myosin heavy chain and a secondary antibody coupled to Alexa 488 after treatment with AZA and/or TSA (a to f) or without treatment (g and h). Nuclei were stained with Hoechst 33258 to locate all cells within a culture (b, d, f, and h). The addition of either drug dramatically increased the number of MHC-positive cells. (B) RT-PCR analysis of the expression of skeletal muscle myogenic factors in mBM-MASCs2 after the addition of AZA, TSA, and AZA/TSA (lanes 1 to 8). RNAs isolated from untreated mBM-MASCs2 (lane 9) and from skeletal muscle tissue (lane 10) were used as negative and positive controls, respectively. Note the lack of Myf5 expression after AZA and AZA/TSA treatment. (C) After treatment with AZA, mBM-MASCs2 were stained with a monoclonal antibody against cTnI and a secondary antibody coupled to Alexa 594 (a and c) in the absence (a) and presence (c) of Hoechst 33258. In panels b and d, treatment with AZA was done with mBM-MASCs2 that contained an α-MHC-eGFP reporter construct. Activation of the α-MHC-eGFP reporter construct is shown under fluorescent light (b) and merged with a corresponding phase-contrast image (d). (D) RT-PCR analysis of the expression of cardiac muscle marker genes in mBM-MASCs1 after the addition of AZA, TSA, and AZA/TSA (lanes 1 to 3). RNAs isolated from untreated mBM-MASCs1 (lane 4) and from heart tissue (lane 5) were used as negative and positive controls, respectively.
DISCUSSION
Adult mesenchymal stem cells do not consist of a phenotypically uniform cell population.
Reports about bone marrow-derived mesenchymal cells that are able to differentiate into numerous different cell types, including cardiac and skeletal muscle cells, neurons, and kidney cells, as well as the description of multipotent adult progenitor cells that are able to form derivatives of all three germ layers, have raised hopes for the use of adult stem cells for therapeutic purposes. It had been suggested that multipotent adult stem cells might be classified by the expression of distinct sets of marker molecules. This concept is derived mainly from highly successful work on hematopoietic stem cells which has led to the identification of pluripotent hematopoietic stem cells, which through a series of developmental events generate cells of the erythroid, myeloid, and lymphoid lineages (reviewed in reference 38). However, it is so far an untested assumption that all adult multipotent progenitor cells consist of a uniform cell type similar to that of pluripotent hematopoietic stem cells that reacts reproducibly upon treatment with certain stimuli. In this study, we have shown that mBM-MASCs might differ in the expressions of two popular stem cell markers, i.e., CD34 and Sca-1, without having major differences in plasticities and differentiation potentials. Minor differences that were found between cell populations seemed due to stochastic deviations rather than to programmed differences between the investigated cells. It also should be kept in mind that the differentiation potential of isolated (stem) cells in vitro might in part be imposed by the cultivation per se and might not necessarily reflect their natural behavior in a stem cell niche.
Although comprehensive surveys of gene activities as obtained by microarray hybridization techniques certainly yield more information than work with single marker genes does (15), they are difficult to interpret, due to the large number of genes affected, the effects of the physiological/metabolic state, and numerous other variables. Most likely, such influences will prevent an unbiased, accurate view of the differentiation potential of a cell. We conclude that an operational definition of mBM-MASCs in response to various inducers defines more precisely the biological potential of a stem cell than does the expression of distinct marker molecules.
Differential induction of cell-type-specific differentiation events by distinct signaling molecules.
The pivotal role of Wnt molecules for somitic myogenesis is well established (9, 22). Although individual Wnt molecules have been implicated in the direct specific expression of either Myf-5 or MyoD, the lack of muscle phenotypes in Wnt knockouts argues against a strict linear relationship (14). In support of this notion, we found that Wnt molecules activate myogenesis in MASCs in a rather promiscuous way. This observation is also backed by our finding of the activation of the canonical Wnt pathway by CA-LEF-stimulated differentiation of adult stem cells, although it has been reported that the activation of the canonical Wnt pathway does not promote differentiation and even inhibits differentiation of embryonic stem cells (30).
We also detected a strong activation of skeletal muscle-specific genes by CDO. CDO has been shown to enhance the differentiation of C2C12 myogenic cells, whereas the expression of dominant negative forms of CDO inhibited differentiation (19). Mice that lack CDO display delayed skeletal muscle development and defective muscle stem cell differentiation, indicating that CDO is part of a positive feedback network that maintains the myogenic transcriptional program (8). Our finding that CDO activates the expression of myogenic regulatory factors and subsequently also of structural muscle proteins nicely fits into the picture. We reason that CDO augments an endogenous propensity of mBM-MASCs to activate myogenic genes, thereby stabilizing the positive feedback loop that ultimately drives myogenesis.
The failure of CDO to induce cardiac muscle-specific genes was in contrast to the ability of Wnt molecules, which stimulated both skeletal and cardiac muscle genes. While the role of Wnt molecules for skeletal muscle development is well established, their function in the development of the cardiac lineage is less clear. It has been proposed that Wnt11, which seems to act via a noncanonical Ca2+- and PKC-dependent pathway, stimulates cardiomyocyte development (10, 24), while other Wnts appear to inhibit cardiogenesis (35). In our experimental setup, we indeed observed a preferential activation of cardiac marker genes by Wnt11 and only a reduced ability of other Wnt molecules to activate cardiomyogenesis, but we observed no suppression.
Wnt molecules activate cardiac and skeletal muscle-specific gene expression via separate intracellular signaling networks.
It is certainly an oversimplification to view skeletal myogenesis as a result of canonical Wnt signaling and cardiogenesis as a result of noncanonical Wnt signaling, in particular since Wnt signals derived from the dorsal ectoderm (Wnt-7a) have also been shown to employ the noncanonical PKC signaling cascade in P19 cells (27). Experiments in chicken embryo demonstrated that Wnt-1 and Wnt-3a induced myogenesis via Frizzled1 and β-catenin/Lef1/T-cell factor signaling (31), while the ability of Wnt11 to induce ectopic cardiogenesis was attributed to the noncanonical Ca2+/PKC-dependent pathway (24). Along the same lines, we demonstrated that the expression of CA-LEF in mBM-MASCs resulted in the activation of skeletal muscle-specific proteins but not in the expression of cardiac muscle-specific genes, with the notable exception of the Nkx-2.5 gene. Inhibition of PKC using the PKC inhibitor bisindolylmaleimide I and the kinase inhibitor staurosporine abrogated Wnt-induced expression of cardiac genes, indicating an important role of noncanonical Wnt signaling in mBM-MASCs for the promotion of the cardiac program.
Plasticity and spontaneous differentiation of MASCs might be enhanced by induction of chromatin remodelling.
Normally, stem cells are maintained in a quiescent state and need specific stimuli for renewal and differentiation. When stem cells differentiate, they have to suppress genes that are incompatible with the upcoming cell type. This might be achieved either by an active control mechanism, which depends on dynamic interactions of regulatory proteins present in the cell, or by a passive mechanism that silences unneeded genes so that they are normally not accessible in a given cell lineage. A passive mechanism might rely on promoter/gene hypermethylation and histone deacetylation (3). Release of the passive control mechanism will result in an increase of gene transcription and in the inappropriate expression of individual genes. In the current study, we observed a spontaneous activation of skeletal and cardiac muscle genes in mBM-MASCs after demethylation and acetylation, indicating that hypermethylation and chromatin compaction by histone deacetylation are important mechanisms by which myogenic gene expression is silenced in stem cells. It seems likely that the forced inhibition of these silencing mechanisms increases cellular plasticity by allowing the spontaneous activation of critical control genes. Such a wave of undirected gene activity might eventually result in the formation of self-maintaining regulatory circuits that enable further differentiation events. The MyoD gene has been shown to be particularly susceptible to such regulation (18). It was therefore no surprise to detect an up-regulation of MyoD but not of Myf5 in AZA- and TSA-treated stem cells. Both acetylation and demethylation were able to initiate myogenic gene activation separately. The combined acetylation and demethylation had no synergistic effects and did not increase the expression of muscle-specific genes further, suggesting that the silencing of the stem cell genome might be overcome by either mechanism, which then dominantly affects the other supplementary mechanism.
Activation of cell-type-specific gene expression pattern versus differentiation to fully functional cells.
Although several groups have reported the ability of mesenchymal stem cells isolated from the bone marrow and other organs to differentiate into numerous specialized cell types or into derivatives of all three germ layers, no rigorous confirmation of the functional abilities of such cells has been made. Repopulation of the stem cell niche, which is a standard procedure in the hematopoietic field, has not been attempted or has failed. Nevertheless, some progress was made in improving the signature of differentiated cells, e.g., by partially restoring dystrophin expression in mdx mice, an animal model of Duchenne's muscular dystrophy, after bone marrow transplantation studies in the mouse (13). However, the latter findings might also be due to the fusion of bone marrow-derived cells to newly forming myotubes (32).
In our hands, mBM-MASC-derived cells that express marker genes characteristic of cardiac and skeletal muscle after induction with different signaling molecules lacked several features of functional muscle cells. They did not form regular cross-striations or show spontaneous contractions, which are typical for cardiomyocytes. If adult stem cells activate only a subset of cell-type-specific genes, then what is the real nature of adult stem cells during normal development? It seems plausible that they simply represent loose ends or leftovers of various progenitor cells that have preserved the remarkable plasticity of their ancestors but stopped serving an important physiological role. It is also possible, however, that the physiological role of adult stem cells has yet to be unveiled. In any case, adult stem cells are remarkably plastic and easily undergo partial differentiation upon induction, despite the failure to acquire fully functional characteristics. Obvious explanations for an incomplete differentiation might be the absence of activating molecules within the cells or the presence of inhibitory factors. We assume that it might be possible to identify missing components and push the faulty differentiation program to completion, essentially reestablishing the active control mechanism which drives any functionally differentiated cell at any given time.
Supplementary Material
Acknowledgments
We thank Katja Zabel and Undine Ziese for expert technical assistance. We are indebted to Jan Kitajewski (Columbia University) for supplying retroviral constructs for different Wnt molecules and to Ken Chien (UCSD) for the α-MHC promoter. We further thank D. Baltimore and B. Wold (Caltech) for the generous gift of the myogenin promoter-eGFP lentiviral reporter construct and Rolf Kemmler (MPI Freiburg) for the CA-LEF construct.
This work was supported by the Max-Planck-Society, the DFG (priority program “stem cells”), and the BMBF. We declare that we have no conflicting commercial interests related to this work.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aoki, M., A. Hecht, U. Kruse, R. Kemler, and P. K. Vogt. 1999. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA 96:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakura, A., P. Seale, A. Girgis-Gabardo, and M. A. Rudnicki. 2002. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159:123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharadwaj, S., and G. L. Prasad. 2002. Tropomyosin-1, a novel suppressor of cellular transformation is downregulated by promoter methylation in cancer cells. Cancer Lett. 183:205-213. [DOI] [PubMed] [Google Scholar]
- 4.Bjornson, C. R., R. L. Rietze, B. A. Reynolds, M. C. Magli, and A. L. Vescovi. 1999. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 283:534-537. [DOI] [PubMed] [Google Scholar]
- 5.Braun, T., and H. H. Arnold. 1996. Myf-5 and myoD genes are activated in distinct mesenchymal stem cells and determine different skeletal muscle cell lineages. EMBO J. 15:310-318. [PMC free article] [PubMed] [Google Scholar]
- 6.Brazelton, T. R., F. M. Rossi, G. I. Keshet, and H. M. Blau. 2000. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290:1775-1779. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila, J., C. Tabin, and R. L. Johnson. 1998. Control of dorsoventral somite patterning by Wnt-1 and beta-catenin. Dev. Biol. 193:182-194. [DOI] [PubMed] [Google Scholar]
- 8.Cole, F., W. Zhang, A. Geyra, J. S. Kang, and R. S. Krauss. 2004. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev. Cell 7:843-854. [DOI] [PubMed] [Google Scholar]
- 9.Cossu, G., and U. Borello. 1999. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 18:6867-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg, C. A., and L. M. Eisenberg. 1999. WNT11 promotes cardiac tissue formation of early mesoderm. Dev. Dyn. 216:45-58. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari, G., G. Cusella-De Angelis, M. Coletta, E. Paolucci, A. Stornaiuolo, G. Cossu, and F. Mavilio. 1998. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528-1530. [DOI] [PubMed] [Google Scholar]
- 12.Floss, T., H. H. Arnold, and T. Braun. 1997. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11:2040-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gussoni, E., Y. Soneoka, C. D. Strickland, E. A. Buzney, M. K. Khan, A. F. Flint, L. M. Kunkel, and R. C. Mulligan. 1999. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401:390-394. [DOI] [PubMed] [Google Scholar]
- 14.Ikeya, M., and S. Takada. 1998. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 125:4969-4976. [DOI] [PubMed] [Google Scholar]
- 15.Ivanova, N. B., J. T. Dimos, C. Schaniel, J. A. Hackney, K. A. Moore, and I. R. Lemischka. 2002. A stem cell molecular signature. Science 298:601-604. [DOI] [PubMed] [Google Scholar]
- 16.Jackson, K. A., T. Mi, and M. A. Goodell. 1999. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA 96:14482-14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, Y., B. N. Jahagirdar, R. L. Reinhardt, R. E. Schwartz, C. D. Keene, X. R. Ortiz-Gonzalez, M. Reyes, T. Lenvik, T. Lund, M. Blackstad, J. Du, S. Aldrich, A. Lisberg, W. C. Low, D. A. Largaespada, and C. M. Verfaillie. 2002. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41-49. [DOI] [PubMed] [Google Scholar]
- 18.Jones, P. A., M. J. Wolkowicz, M. A. Harrington, and F. Gonzales. 1990. Methylation and expression of the Myo D1 determination gene. Philos. Trans. R. Soc. London B 326:277-284. [DOI] [PubMed] [Google Scholar]
- 19.Kang, J. S., P. J. Mulieri, C. Miller, D. A. Sassoon, and R. S. Krauss. 1998. CDO, a robo-related cell surface protein that mediates myogenic differentiation. J. Cell Biol. 143:403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 21.Mennerich, D., and T. Braun. 2001. Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J. 20:7174-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munsterberg, A. E., J. Kitajewski, D. A. Bumcrot, A. P. McMahon, and A. B. Lassar. 1995. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 9:2911-2922. [DOI] [PubMed] [Google Scholar]
- 23.Neuhaus, P., S. Oustanina, T. Loch, M. Kruger, E. Bober, R. Dono, R. Zeller, and T. Braun. 2003. Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol. Cell. Biol. 23:6037-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandur, P., M. Lasche, L. M. Eisenberg, and M. Kuhl. 2002. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418:636-641. [DOI] [PubMed] [Google Scholar]
- 25.Polesskaya, A., P. Seale, and M. A. Rudnicki. 2003. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113:841-852. [DOI] [PubMed] [Google Scholar]
- 26.Prockop, D. J. 1997. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71-74. [DOI] [PubMed] [Google Scholar]
- 27.Ridgeway, A. G., H. Petropoulos, S. Wilton, and I. S. Skerjanc. 2000. Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 275:32398-32405. [DOI] [PubMed] [Google Scholar]
- 28.Rietze, R. L., H. Valcanis, G. F. Brooker, T. Thomas, A. K. Voss, and P. F. Bartlett. 2001. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 412:736-739. [DOI] [PubMed] [Google Scholar]
- 29.Santa Maria, L., C. V. Rojas, and J. J. Minguell. 2004. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp. Cell Res. 300:418-426. [DOI] [PubMed] [Google Scholar]
- 30.Sato, N., L. Meijer, L. Skaltsounis, P. Greengard, and A. H. Brivanlou. 2004. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10:55-63. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt, M., M. Tanaka, and A. Munsterberg. 2000. Expression of (beta)-catenin in the developing chick myotome is regulated by myogenic signals. Development 127:4105-4113. [DOI] [PubMed] [Google Scholar]
- 32.Sherwood, R. I., J. L. Christensen, I. M. Conboy, M. J. Conboy, T. A. Rando, I. L. Weissman, and A. J. Wagers. 2004. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119:543-554. [DOI] [PubMed] [Google Scholar]
- 33.Theise, N. D., S. Badve, R. Saxena, O. Henegariu, S. Sell, J. M. Crawford, and D. S. Krause. 2000. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 31:235-240. [DOI] [PubMed] [Google Scholar]
- 34.Toma, J. G., M. Akhavan, K. J. Fernandes, F. Barnabe-Heider, A. Sadikot, D. R. Kaplan, and F. D. Miller. 2001. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3:778-784. [DOI] [PubMed] [Google Scholar]
- 35.Tzahor, E., and A. B. Lassar. 2001. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 15:255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurmser, A. E., K. Nakashima, R. G. Summers, N. Toni, K. A. D'Amour, D. C. Lie, and F. H. Gage. 2004. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430:350-356. [DOI] [PubMed] [Google Scholar]
- 37.Zhao, P., and E. P. Hoffman. 2004. Embryonic myogenesis pathways in muscle regeneration. Dev. Dyn. 229:380-392. [DOI] [PubMed] [Google Scholar]
- 38.Zon, L. I. 2001. Hematopoiesis: a developmental approach. Oxford University Press, New York, N.Y.
- 39.Zuk, P. A., M. Zhu, H. Mizuno, J. Huang, J. W. Futrell, A. J. Katz, P. Benhaim, H. P. Lorenz, and M. H. Hedrick. 2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7:211-228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.