Abstract
Alteromonas sp. strain O-7 secretes chitinase A (ChiA), chitinase B (ChiB), and chitinase C (ChiC) in the presence of chitin. A gene cluster involved in the chitinolytic system of the strain was cloned and sequenced upstream of and including the chiA gene. The gene cluster consisted of three different open reading frames organized in the order chiD, cbp1, and chiA. The chiD, cbp1, and chiA genes were closely linked and transcribed in the same direction. Sequence analysis indicated that Cbp1 (475 amino acids) was a chitin-binding protein composed of two discrete functional regions. ChiD (1,037 amino acids) showed sequence similarity to bacterial chitinases classified into family 18 of glycosyl hydrolases. The cbp1 and chiD genes were expressed in Escherichia coli, and the recombinant proteins were purified to homogeneity. The highest binding activities of Cbp1 and ChiD were observed when α-chitin was used as a substrate. Cbp1 and ChiD possessed a chitin-binding domain (ChtBD) belonging to ChtBD type 3. ChiD rapidly hydrolyzed chitin oligosaccharides in sizes from trimers to hexamers, but not chitin. However, after prolonged incubation with large amounts of ChiD, the enzyme produced a small amount of (GlcNAc)2 from chitin. The optimum temperature and pH of ChiD were 50°C and 7.0, respectively.
Chitin, an insoluble linear β-1,4-linked polymer of N-acetylglucosamine (GlcNAc), is the second most abundant polymer in nature. This polysaccharide is particularly an important nutrient source for maintaining the ecosystem in the marine environment (7). Chitinolytic marine bacteria play a critical role in the process of recycling chitinous materials such as the exoskeletons of crustaceans and insects. If the insoluble form of chitin could not be returned to the ecosystem in a biologically usable form, the marine environment would be completely depleted of a carbon and nitrogen source in a relatively short time (39). To degrade chitin, chitinolytic microorganisms produce two classes of enzymes: chitinases (EC 3.2.1.14) and β-N-acetylglucosaminidases (GlcNAcases; EC 3.2.1.30). Chitinases hydrolyze chitin to soluble oligosaccharides, mainly chitobiose, which are further hydrolyzed to GlcNAc by GlcNAcases. Finally, the degradation products, mainly GlcNAc, are then taken up by the cells as a carbon and nitrogen source.
Alteromonas sp. strain O-7 is a gram-negative, flagellated, motile, and aerobic rod-shaped bacterium of marine origin (30). This strain was isolated from a sediment sample at Sagami Bay in Japan as an efficient producer of chitinolytic enzymes (30). We have been studying the chitin degradation system of the strain as a model for defining the various components involved in chitin utilization. It was made clear that the chitinolytic system of the strain consists of at least four chitinases (Chi85, ChiA, ChiB, and ChiC) and three β-N-acetylglucosaminidases (GlcNAcaseA, GlcNAcaseB, and GlcNAcaseC). The genes for these enzymes have been cloned and characterized previously to clarify the role of individual enzymes in the chitinolytic system of Alteromonas sp. strain O-7 (31–35). In addition, we recently found a novel transglycosylative enzyme (Hex99) which synthesized β-(1→6)-(GlcNAc)2 from β-(1→4)-(GlcNAc)2 and demonstrated that β-(1→6)-(GlcNAc)2 was one of the smallest molecules to induce chitinase production in the microorganism (36). Among three chitinases, ChiA, which is the truncated form of Chi85, is the major enzyme (∼70% of total chitinase activity) in the culture supernatant (36). Chi85 is a modular enzyme with three domains. Its catalytic domain is flanked by an N-terminal domain of unknown function and a C-terminal chitin-binding domain (31). The C-terminal portion of Chi85 is cleaved by an extracellular protease(s) from the strain to give the truncated form of Chi85, termed ChiA, which is composed of an N-terminal domain of unknown function and a catalytic domain. Recently, sequence analysis of the upstream region of the chiA gene revealed a gene cluster consisting of three complete open reading frames (ORFs) organized in the order chiD, cbp1, and chiA. In the present study, we report the complete nucleotide sequence and polypeptide analysis of an 8.2-kb region encoding a chitinase-like enzyme (ChiD) with high activity only toward chitooligosaccharides, a novel chitin-binding protein (Cbp1), and ChiA. Furthermore, we have overexpressed ChiD and Cbp1 in Escherichia coli and investigated the roles of both proteins in the chitin degradation system of this bacterium.
MATERIALS AND METHODS
Strains, vectors, and culture media.
Alteromonas sp. strain O-7 was grown at 27°C in Bacto Marine Broth 2216 (Difco) and was used as the source of chromosomal DNA (31). The E. coli strains employed in this study were JM109 and BL21(DE3). The vectors used were pUC18, pUC19 (Takara Biomedicals, Shiga, Japan), pMOSBlue (Amersham Pharmacia Biotech), pGEX (Amersham Pharmacia Biotech), and pET-20b(+) (Novagen). E. coli cells were cultured in Luria broth supplemented with appropriate antibiotics at 37°C.
General recombinant DNA techniques.
Agarose gel electrophoresis, plasmid DNA preparation, transformation of E. coli, and Southern hybridization were performed, and restriction enzymes and T4 DNA ligase were used, as described by Sambrook and Russell (23). Restriction enzymes and other modifying enzymes were purchased from Toyobo (Osaka, Japan).
Cloning of the 5′ upstream region of cbp1.
We carried out cloning of the 5′ upstream region of the cbp1 gene by the DNA-probing method with an 0.30-kb EcoRI-SphI fragment of pCHI997 encoding Cbp1 and ChiA as a probe. The fragment was labeled with alkaline phosphatase according to the manufacturer’s instructions (AlkPhos DIRECT; Amersham Pharmacia Biotech). Chromosomal DNA of strain O-7 was digested with BamHI and SphI and electrophoresed on an 0.6% agarose gel. The fragments of 1.8-kb fractions were excised from the gel and purified with a Sephaglas BandPrep kit (Pharmacia). These were ligated into the dephosphorylated BamHI-SphI site of pUC18, and the recombinant plasmids were introduced into competent E. coli JM109. The library was screened by colony hybridization with the labeled probe. Hybridization and washing of the transfer membranes (Hybond-N+; Amersham Pharmacia Biotech) were performed as recommended by the manufacturer. To clone the 5′ upstream region of the 1.8-kb BamHI-SphI fragment, the second colony hybridization was performed using an 0.6-kb BamHI-PstI fragment as a probe.
Nucleotide sequencing.
DNA sequencing was performed by the dideoxy chain termination method (24) using a Thermo Sequenase fluorescence-labeled primer cycle sequencing kit (Amersham Pharmacia Biotech) on a DNA sequencer (Hitachi SQ3000). Sequence data were analyzed with DNASIS software (Hitachi Software Engineering Co. Ltd., Yokohama, Japan).
Construction of expression plasmids.
The expression plasmid pET-ChiD, coding for ChiD, was constructed as follows. Two oligonucleotide primers, 5′-CGGAGCCATTTTCTGCGCGAGCTCATATGC-3′ and 5′-CATTTATTATCTCGAGGTTGCAAGCCTTGG-3′, were synthesized, which were modified to contain SacI and XhoI recognition sites to facilitate cloning in frame into pET-20b(+). The chiD gene was amplified by PCR with the primers with pCHI997.3 as the template. PCR was performed for 30 cycles consisting of 94°C for 30 s, 40°C for 2 s, and 74°C for 30 s. The amplified DNA was digested by SacI and XhoI, and the resulting fragment (3 kb) was inserted into the corresponding sites of pET-20b(+). On the other hand, a 1,380-bp fragment of cbp1, coding for Cbp1, was prepared by PCR with plasmid pCHI997 as the template and the primers 5′-ATTTCAAGCGGAATTCAGCT-3′ and 5′-TTAATTTGGGTCGACTTTAAATTG-3′. The amplified DNA was digested by SalI and XhoI, and the resulting fragment was inserted in frame into the glutathione S-transferase (GST) fusion protein expression vector pGEX-5X-3 (Amersham Pharmacia Biotech). The resulting plasmid was designated pGEX-Cbp1. The 5′ and 3′ parts of cbp1 were also amplified by PCR in the same manner as that of pGEX-Cbp1. Four oligonucleotides (5′-ATTTCAAGCGGAATTCAGCT-3′, 5′-TGGCGTGCCTCTCGAGGTTATTGT-3′, 5′-TTAATTTGGGTCGACTTTAAATTG-3′, and 5′-TTAATTTGGGTCGACTTTAAATTG-3′) were used as primers for PCR. The PCR products of the 5′ and 3′ parts of cbp1 were inserted in frame between EcoRI and XhoI sites and EcoRI and SalI sites of pGEX-5X-3, respectively. The resulting plasmids encoding the N-terminal (residues Ser25 to Thr227) and C-terminal (residues Ala278 to Ile475) regions of Cbp1 were designated pGEX-CbpΔC and pGEX-CbpΔN, respectively. All PCR amplifications described above were performed for 30 cycles consisting of 94°C for 30 s, 40°C for 2 s, and 74°C for 30 s. The nucleotide sequences of the junctions between vectors and inserts and the whole amplified DNA were confirmed with a Thermo Sequenase cycle sequencing kit with fluorescently labeled primers.
Purification of recombinant proteins.
E. coli BL21(DE3) cells harboring pET-ChiD were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at the mid-exponential growth phase and further incubated for 4 h at 37°C. Cells were harvested by centrifugation, washed with 50 mM Tris-HCl buffer containing 1 mM EDTA (pH 7.5), and resuspended in the buffer. The cells were disrupted by sonication, and the lysate was centrifuged at 10,000 × g for 20 min. The pellet containing insoluble ChiD was solubilized with 50 mM Tris-HCl buffer (pH 7.5) containing 6 M guanidine hydrochloride. The solution was centrifuged at 10,000 × g for 20 min and dialyzed against 50 mM Tris-HCl buffer (pH 7.5) for 10 h at 4°C. The supernatant was loaded onto a DEAE-Toyopearl 650 M column equilibrated with 50 mM Tris-HCl buffer (pH 7.5) and eluted with a linear gradient of 0 to 1.0 M NaCl in the same buffer at a flow rate of 2 ml/min. The active fractions were combined and concentrated by ultrafiltration with a Q 0100 membrane. The concentrated sample was applied to a Sephadex G-100 column equilibrated with the buffer containing 0.1 M NaCl. The active fractions were collected and chromatographed with a linear gradient of NaCl with a MonoQ fast protein liquid chromatography column. Active fractions were combined and used as the purified enzyme. On the other hand, each of the fusion proteins was purified from E. coli BL21 lysate by affinity chromatography with glutathione-Sepharose 4B. The purified fusion proteins were treated with PreScission protease (Amersham Pharmacia Biotech) for 12 h at 24°C to obtain Cbp1, Cbp1ΔC, and Cbp1ΔN.
Enzyme assay.
Chitinase activity was measured as described previously with colloidal chitin or p-nitrophenyl-N,N′-diacetylchitobiose [pNP-(GlcNAc)2] as a substrate (31, 36). Enzymatic hydrolysis of chitin or chitooligosaccharides from dimers to hexamers was analyzed by thin-layer chromatography (TLC). The reaction mixture contained 50 mM Tris-HCl buffer (pH 7.0), 1 mg of substrate, and enzyme solution in a total volume of 20 μl. The reaction mixtures were incubated at 50°C for 30 min, and 3 μl of the reaction mixture was applied to a silica gel plate (Kiselgel 60; Merck Co., Berlin, Germany) and chromatographed in isopropyl alcohol-ethanol-water (5:2:1 [vol/vol]). The plates were developed by spraying the plates with 10% sulfuric acid in ethanol followed by heating at 120°C for 10 to 20 min to detect dark spots.
Chitin binding study.
Binding assays were carried out by adding 5 μg of purified Cbp1, Cbp1ΔC, Cbp1ΔN, or ChiD to 1 mg each of α-chitin, β-chitin, chitosan, and crystalline cellulose (Avicel) in 0.3 ml of 50 mM Tris-HCl buffer (pH 7.0) containing 0.1 M NaCl in 1.5-ml microcentrifuge tubes. Samples were incubated at 27°C for 1 h with mixing every 10 min and then centrifuged at 24,650 × g for 5 min. The unadsorbed protein concentration in the supernatant was determined from the absorbance at 280 nm. The extinction coefficient for Cbp1, Cbp1ΔC, Cbp1ΔN, and ChiD was predicted from the tryptophan, tyrosine, and cysteine contents of the proteins (6). The bound protein concentration was determined from the difference between the initial protein and unadsorbed protein concentrations.
Effect of Cbp1 on chitinase activity.
Chi85 and ChiA were purified by a Hitrap metal chelating column (Amersham Pharmacia Biotech) from extracts of E. coli carrying pETChi85 and pETChiA, respectively. The expression plasmids pETChi85 and pETChiA were constructed using plasmid pCHI997 (31) as a template. A 2,484-bp DNA fragment, coding for Chi85, was prepared by PCR with primers F1 (5′-AGTGGCGGAGATCTTGCAG-3′) and R1 (5′-ATAAGCTTGTTAGTTACTGCC-3′), and a 1,698-bp DNA fragment, coding for ChiA, was prepared by PCR with primers F1 and R2 (5′-CATTGCATTTAGAAGCTTGCC-3′). PCR was performed for 30 cycles consisting of 94°C for 15 s, 58°C for 30 s, and 68°C for 2 min. Two amplified DNA fragments were digested by BglII and HindIII, and each of the resulting fragments was inserted in frame into pET-20b(+). Various concentrations of Cbp1 were added to powdered chitin or colloidal chitin (chitin EX from crab shells; Funakoshi Co., Ltd., Tokyo, Japan) suspended with 50 mM Tris-HCl buffer (pH 7.5) containing 0.02% NaN3. The suspension was preincubated for 1 h in a 1.5-ml plastic Eppendorf tube. Purified Chi85 (5 μg) or ChiA (5 μg) was then added to a total volume of 1 ml, and the reaction mixture was further incubated at 37°C for 16 h. Samples were taken at the appropriate intervals. The reaction was terminated by cooling the mixture on ice and centrifuging it at 20,000 × g for 30 min. Reducing sugar in the supernatant was measured according to the method described in a previous paper (31).
N-terminal amino acid sequence, protein assay, and SDS-PAGE.
The amino acid sequence was analyzed by an Applied Biosystems Procise 491 HT protein sequencer. Protein was assayed by the method of Bradford (1) with bovine serum albumin as a standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described before (31).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB063629.
RESULTS
Cloning of the gene cluster.
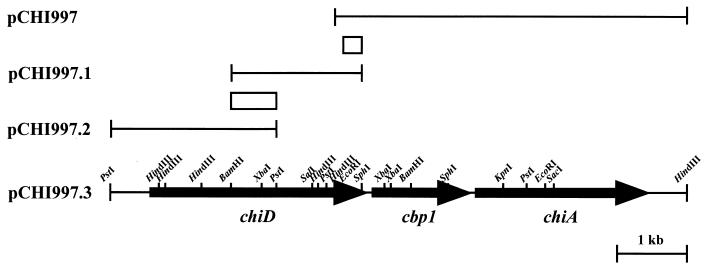
Previous studies of ChiA in Alteromonas sp. strain O-7 led to the identification and characterization of chiD and cbp1 genes. Plasmid pCHI997 contains a 5.0-kb HindIII fragment of the Alteromonas sp. strain O-7 chromosome carrying the chitinase gene chiA (Fig. 1). Sequence analysis of the 1.9-kb region immediately upstream of chiA revealed a complete ORF, designated cbp1, and a partial ORF encoding a protein which showed sequence similarity to the chitin-binding domain commonly found in Chi85, ChiB, and ChiC. Therefore, cloning of the 5′ upstream region of the cbp1 gene was performed by colony hybridization using the 0.3-kb EcoRI-SphI fragment as a probe as shown in Fig. 1. The results of Southern hybridization against total DNA digested with various endonucleases showed that the probe strongly hybridized with the 1.8-kb BamHI-SphI fragment (data not shown). These DNA fragments were ligated into the corresponding sites of pUC18 and introduced into E. coli JM109. Among 1,400 transformants, only one clone (pCHI997.1) which hybridized with the probe was isolated by colony hybridization (Fig. 1). Analyses by restriction enzyme digestion and sequencing of the fragment revealed that the insert of pCHI997.1 and that of pCHI997 shared a 0.3-kb EcoRI-SphI region. However, analysis of the entire nucleotide sequence of pCHI997.1 indicated that the 5′ upstream region of the gene was deleted. Then, the 5′ upstream region of the insert of pCHI997.1, designated pCHI997.2, was further cloned by the second colony hybridization using the 0.6-kb BamHI-PstI fragment as a probe (Fig. 1). The 5.0-kb HindIII fragment of pCHI997, 1.8-kb BamHI-SphI fragment of pCHI997.1, and 2.4-kb PstI-PstI fragment of pCHI997.2 were ligated together, and the resulting 8.2-kb fragment was inserted into the corresponding sites of pUC18. The resulting plasmid was named pCHI997.3 (Fig. 1).
FIG. 1.
Restriction map of chiD, cbp1, and chiA. The hybridization probes are represented by the boxes. The arrows indicate the ORFs and directions of transcription.
Nucleotide sequence analysis.
The nucleotide sequence of the gene cluster was determined. The overall G+C content of the 8.2-kb fragment from pCHI997.3 was 40.5%, which is compatible with the G+C content typically found for strains of the genus Alteromonas (30). Three ORFs (chiD, cbp1, and chiA) were encoded on the same strand and were preceded by plausible ribosome binding sites. There are only 55 nucleotides between the TTA termination codon of cbp1 and the ATG initiation codon of chiA and 45 nucleotides between chiD and cbp1.
The cbp1 gene consists of 1,428 nucleotides encoding a protein of 475 amino acids with a predicted molecular mass of 52,082 Da. The deduced N-terminal 26-amino-acid sequence showed the typical features of signal peptides, which are composed of a positively charged region, a hydrophobic region, and a signal sequence cleavage site. From the characterization of the cleavage sites, it is presumed that the putative site of cleavage might be between alanine 26 and histidine 27, which is compatible with the −3, −1 rule of von Heijne (37). Upstream of cbp1, an ORF designated chiD was found, and it encoded a protein of 1,037 amino acids with a deduced molecular mass of 112,178 Da, which showed similarity with family 18 bacterial chitinases (9–11). In the N-terminal portion, a hydrophobic core region that seems to be functional as a signal sequence was found. This 26-residue signal peptide ends with a Ser-Tyr-Ala recognition site for cleavage by the signal peptidase.
Structural features of Cbp1 and ChiD.
The BLAST analysis program was used to compare Cbp1 and ChiD with proteins in databases. The N-terminal region of Cbp1 (residues His27 to Ile224) had similarity with chitinase B from Pseudoalteromonas strains (66% identity) (29), a spindle-like protein from Choristoneura fumiferana (33% identity) (14), spheroidin-like protein from Orgyia pseudotsugata nuclear polyhedrosis virus (33% identity) (8), cellulose-binding protein from Streptomyces halstedii (32% identity) (5), and spindle body protein from Heliothis armigera poxvirus (31% identity) (3) (Fig. 2). On the other hand, the C-terminal region (residues Thr280 to Ile475) revealed homologies to ChiB from Pseudoalteromonas sp. strain S9 (43% identity) (29), Chi85 from Alteromonas sp. strain O-7 (39% identity) (31), ChiA from Aeromonas hydrophila (33% identity) (22), ChiB from Vibrio alginolyticus (33% identity) (19), and ChiA from Aeromonas caviae (32% identity) (26) (Fig. 3). Furthermore, the residues 435 to 475 of the region showed high sequence similarity with a chitin-binding domain (ChtBD) classified as type 3 by the National Center for Biotechnology Information conserved domain database (http://www.ncbi.nlm.nih.gov/Structure/cdd/-cdd.shtml). The serine-, threonine-, and proline-rich region (TSTGTPP) was found between the N- and C-terminal regions, and it was similar to the linker or hinge region of microbial enzymes. These results indicate that Cbp1 is a putative chitin-binding protein composed of two discrete functional regions.
FIG. 2.
Sequence alignment of the N-terminal regions of Cbp1 and the other proteins. The residue number of the first amino acid in each line is shown on the left. Identical amino acid residues are indicated by boldface letters. Cbp1, chitin-binding protein from Alteromonas sp. strain O-7; ChiB, chitinase B from Pseudoalteromonas sp. strain S91; SPIN, spindle-like protein from C. fumiferana; SPHE, spheroidin-like protein from O. pseudotsugata; CBP, cellulose-binding protein from S. halstedii; SPINB, spindle body protein from H. armigera.
FIG. 3.
Sequence alignment of the C-terminal regions of Cbp1 and the other proteins. The residue number of the first amino acid in each line is shown on the left. Identical amino acid residues are indicated by boldface letters. Cbp1, chitin-binding protein from Alteromonas sp. strain O-7; ChiBp, chitinase B from Pseudoalteromonas sp. strain S91; Chi85, chitinase 85 from Alteromonas sp. strain O-7; ChiAh, chitinase A from A. hydrophila; ChiBv, chitinase B from V. alginolyticus; ChiAc, chitinase A from A. caviae. The box indicates a type 3 chitin-binding domain.
The deduced amino acid sequence of ChiD showed sequence homology with enzymes classified in family 18 of glycosyl hydrolases, such as ChiA from Pseudoalteromonas sp. strain S91 (64% identity) (29), Chi92 from Streptomyces olivaceoviridis (56% identity) (13), ChiA from Arthrobacter sp. strain TAD20 (45% identity) (15), chitodextrinase (EndoI) from Vibrio furnissii (12) (44% identity), ChiA from Clostridium paraputrificum (35% identity) (18), and ChiA from Pyrococcus kodakaraensis (29% identity) (28). Two highly conserved regions (S-X-G-G and D-X-D-X-E) were identified in the catalytic domain of chitinases classified as family 18 glycosyl hydrolases (38). The consensus sequences S-X-G-G and D-X-D-X-E indicate substrate-binding and active sites, respectively, where X represents hydrophobic amino acids. These consensus sequences (amino acids 445 to 448 and 503 to 507, respectively) were also found in the catalytic center of ChiD. Furthermore, two domains, which were homologous to ChtBD type 3, were also found in the N-terminal (amino acids 28 to 82) and C-terminal (amino acids 987 to 1037) regions of ChiD.
Expression and purification of Cbp1 and ChiD.
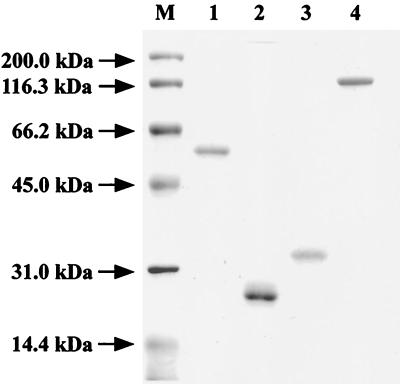
To elucidate the function of Cbp1 in the chitinolytic system of Alteromonas sp. strain O-7, the genes encoding Cbp1, Cbp1ΔC, and Cbp1ΔN were constructed by using the GST-fusion protein expression vector pGEX-5X-3 as described in Materials and Methods. Each of the hybrid genes produced the directing protein in E. coli BL21 cells. The fusion proteins, GST-Cbp1, GST-Cbp1ΔC, and GST-Cbp1ΔN, were purified by affinity chromatography with glutathione-Sepharose 4B (data not shown). The purified proteins were treated with PreScission protease, and then Cbp1, Cbp1ΔC, and Cbp1 ΔN were purified by glutathione-Sepharose 4B (Fig. 4). The molecular mass of Cbp1ΔC (24 kDa) calculated from the amino acid sequence was in reasonable agreement with that estimated by SDS-PAGE. However, the molecular masses of Cbp1 (60 kDa) and Cbp1ΔN (33 kDa) estimated by SDS-PAGE were higher than their theoretical values (Cbp1, 50 kDa; Cbp1ΔN, 22 kDa) despite the confirmation of their nucleotide sequences and N-terminal amino acid sequences. The strange mobility of these proteins on SDS-PAGE might be due to an unusual structural property of the C-terminal domain of Cbp1.
FIG. 4.
SDS-PAGE of Cbp1, Cbp1ΔC, Cbp1ΔN, and ChiD. Lanes: M, molecular size standards; 1, Cbp1; 2, Cbp1ΔC; 3, Cbp1ΔN; 4, ChiD.
On the other hand, the chiD gene was overexpressed by using plasmid pET-20b(+) introducing a synthetic His6 tag sequence. Most of ChiD was produced as the insoluble pellet in E. coli BL21(DE3) cells after 4 h of induction with IPTG. Further induction decreased the productivity of ChiD. The cells were disrupted by sonication, and the protein pellet was solubilized in 6 M guanidine hydrochloride. The solubilized ChiD was dialyzed against 50 mM Tris-HCl buffer, pH 7.0, and purified by successive column chromatographies, as described in Materials and Methods (Fig. 4). The molecular mass (109,242 Da) of ChiD calculated from the deduced amino acid sequence without signal peptide is in reasonable agreement with the 110 kDa estimated by SDS-PAGE (Fig. 4) and gel filtration, respectively. Purified ChiD was used for further enzymatic characterization.
Characterization of Cbp1.
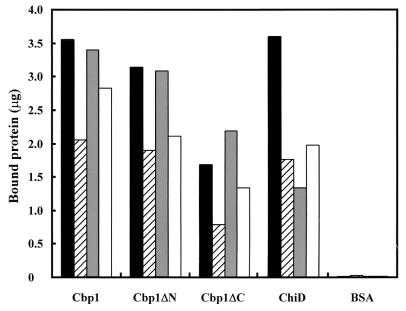
Judging from the structural features of Cbp1, the protein, lacking chitinase activity, is a putative chitin-binding protein consisting of two discrete regions. To examine the function of each region of Cbp1, three purified proteins, Cbp1, Cbp1ΔC, and Cbp1ΔN, were used for chitin binding study. As shown in Fig. 5, the highest binding activity of Cbp1 was observed when α-chitin was used as a substrate, followed by Avicel. However, the binding activity to β-chitin was approximately one-half of that to α-chitin. On the other hand, the binding of Cbp1ΔN to insoluble polysaccharides was similar to that of Cbp1, but Cbp1ΔC exhibited relatively weak affinity to all insoluble polysaccharides examined in this experiment. These results indicate that the C-terminal region mainly contributes to the binding of Cbp1 to α-chitin and Avicel. However, the function of the N-terminal region of Cbp1 is unclear. Binding activity of Cbp1 was not affected over a broad range of pH values (pH 5 to 10) and temperatures (20 to 60°C). Acidic pH values (pH 4.0 or less) and high temperatures of 70°C or higher led to the precipitation of the proteins (data not shown). Since Cbp1 was shown to bind with α-chitin, the effect of Cbp1 on the hydrolysis of α-chitin by defined amounts of ChiA or Chi85 was examined. However, the addition of Cbp1 (10 to 150 μg) to the reaction mixture had no effect on the chitin hydrolysis by ChiA or Chi85.
FIG. 5.
Binding of Cbp1, Cbp1ΔN, Cbp1ΔC, and ChiD to polysaccharides. Black bars, α-chitin; striped bars, β-chitin; gray bars, Avicel; white bars, chitosan. The reaction mixtures were incubated at 27°C for 1 h. Bovine serum albumin (BSA; 5 μg) was used as a control. Data are the averages of three independent experiments.
Characterization of ChiD.
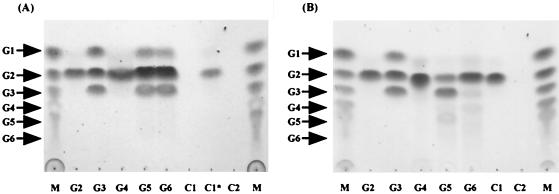
To clarify the role of ChiD in the chitin degradation system of the strain, its enzymatic properties were examined. An initial characterization of ChiD was performed using pNP-(GlcNAc)2 and pNP-(GlcNAc)3, chromogenic substrates for chitinase. pNP-(GlcNAc)2 was a good substrate for ChiD, while only negligible activity was obtained with pNP-(GlcNAc)3, indicating that this compound was mainly cleaved into pNP-(GlcNAc) and (GlcNAc)2. When pNP-(GlcNAc)2 was used as a substrate, the optimum pH of the enzyme was 7.0 and the optimum temperature was 50°C. The experiments described below were therefore performed in 50 mM Tris-HCl buffer (pH 7.0) at 50°C. Since the amino acid sequence of ChiD showed significant similarity to that of bacterial chitinases, we compared the properties of ChiD with those of ChiA using natural substrates. When ChiD was incubated with colloidal chitin for 30 min, TLC analysis demonstrated that the enzyme did not produce (GlcNAc)2 or any other oligosaccharides. However, after prolonged incubation (36 h) using 10 times the amount of ChiD in the standard assay conditions, small amounts of (GlcNAc)2 were detected on a TLC plate (Fig. 6, lane C1*). On the other hand, ChiA rapidly hydrolyzed chitin to give (GlcNAc)2 (Fig. 6). When oligosaccharides from dimers to hexamers were used as a substrate, ChiD hydrolyzed oligosaccharides from (GlcNAc)3 to (GlcNAc)6 under the standard assay conditions but not (GlcNAc)2. The enzyme hydrolyzed (GlcNAc)3 to (GlcNAc)2 and GlcNAc and hydrolyzed (GlcNAc)4 to (GlcNAc)2 and GlcNAc. The (GlcNAc)5 and (GlcNAc)6 were hydrolyzed to (GlcNAc)3, (GlcNAc)2, and GlcNAc. These results suggest that ChiD, unlike chitinases, prefers chitin oligosaccharides [(GlcNAc)3 to (GlcNAc)6] or longer oligosaccharides to chitin as a substrate and that the enzyme hydrolyzes the substrates by an endo-type mechanism. On the other hand, ChiA hydrolyzed chitin oligosaccharides from trimers to hexamers and gave chitobiose as the major product (Fig. 6).
FIG. 6.
TLC of the hydrolysis products of chitin and oligosaccharides by the purified ChiD (A) or ChiA (B). Lanes M show chitooligosaccharides from GlcNAc (G1) to (GlcNAc)6 (G6). For the other lanes, G2, G3, G4, G5, G6, and chitin (C1) were hydrolyzed with the defined amounts of ChiD or ChiA. Lanes C2, chitin without enzyme. The reaction mixture contained 50 mM Tris-HCl buffer (pH 7.0), 10 μg of enzyme, and 1 mg of substrate. Incubations were performed at 50°C for 30 min. In lane C1*, incubations were performed at 50°C for 36 h using 100 μg of ChiD.
DISCUSSION
To utilize chitin as a sole source of carbon, many bacteria usually produce several chitinases to effectively hydrolyze chitin found in nature. Zymogram analysis demonstrated that a marine bacterium, Alteromonas sp. strain O-7, produced at least four chitinases derived from chiA, chiB, and chiC genes (36). Of these, ChiA, a truncated form of Chi85, plays a major role in chitin degradation by the strain. In the present study, we cloned chiD and cbp1 genes located in the region immediately upstream of chiA and investigated the roles of their translated products in the chitinolytic system of the strain.
Analysis of the deduced amino acid sequence of Cbp1 showed that the protein is composed of two distinct regions. The N-terminal region comprising 199 amino acids exhibited high sequence homology with spheroidin and fusolin found in entomopoxviruses (EPVs) associated with insect diseases. Most of the EPVs form two types of occlusion bodies called spheroid and spindle (20). The major protein of spheroid is called spheroidin, while the protein of spindle is called fusolin. Recently, Mitsuhashi et al. reported that Anomala cuprea EPV spindle bodies enhanced Bombyx mori nucleopolyhedrovirus infection of B. mori up to 104-fold (16). However, Cbp1 had no effect on B. mori nucleopolyhedrovirus infection of B. mori at the concentration of 30 μg/insect (unpublished data). Although the N-terminal region of Cbp1 showed weak affinity to α-chitin, the role in the chitinolytic system of Alteromonas sp. strain O-7 is less clear. On the other hand, the C-terminal region of Cbp1 exhibited high affinity to α-chitin and Avicel. In this region, the residues 435 to 475 showed high sequence similarity with the chitin-binding domain (ChtBD) belonging to type 3. Recently, ChtBDs have been classified into types 1, 2, and 3, based on amino acid sequence similarities. The cellulose-binding domain of endoglucanase Z (CBDEGZ) (2), which is a member of CBD family V, has been classified as ChtBD type 3 since CBDEGZ exhibits no similarity with any known CBD but shows sequence similarity with ChtBDs found in many bacterial chitinases. The consensus sequence of ChtBD type 3 (42 amino acid residues) is AWAAGTVYTAGDVVSYNGKVYKAKWWTQGEEPGSSSGPWQLV. Brun et al. proposed that ChtBDs shown in Fig. 3 possess the highly conserved stWWst motif (AKWWTQ motif), where s and t represent small residues and turn-like residues, respectively (2). Furthermore, they proposed that the stWWst motif was essential for the CBD-cellulose interactions based on the three-dimensional structure of the 62-amino-acid C-terminal CBDEGZ (2). Two aromatic rings of CBDEGZ are thought to stack directly against the pyranose rings of cellulose, providing a hydrophobic driving force for the binding. Therefore, it seems that Cbp1ΔN binds α-chitin and Avicel in the same manner as that of CBDEGZ, since these polysaccharides are structurally similar. Chitin-binding proteins have been isolated from marine and terrestrial microorganisms. For marine bacteria such as Vibrio harveyi (17) and V. alginolyticus (21), it was reported elsewhere that chitin-binding proteins located in the outer membrane are required for attachment of bacterial cells to the surface of chitin that is abundant in the marine environment. On the other hand, in soil bacteria such as S. olivaceoviridis (25), Serratia marcescens (27), and Pseudomonas aeruginosa (4), the physiological function of these secreted chitin-binding proteins in the chitin degradation system is still not clear. Cbp1 is one of the proteins secreted by Alteromonas sp. strain O-7 (unpublished data) and bound strongly to α-chitin. Thus, we examined the effect of Cbp1 on the hydrolysis of α-chitin by Chi85 or ChiA. However, the addition of various amounts of Cbp1 to the enzyme mixture had no effect on the hydrolysis efficiency of both enzymes. An interesting hypothesis to test would be whether Cbp1 might be needed under the formation of biofilm on natural biodegradable substrata such as chitin, an important component of the exoskeletons of crustaceans in the marine environment.
The gene encoding ChiD was cloned, sequenced, and overexpressed in E. coli carrying pET-ChiD. The deduced amino acid sequence of ChiD revealed a multidomain structure. The catalytic domain flanked by the N- and C-terminal ChtBDs belonging to type 3 was homologous to chitinases classified as family 18 of glycosyl hydrolases. Comparison of the amino acid sequence of ChiD with those of several chitinases does not reveal any salient structural differences. In fact, two consensus sequences, S-X-G-G and D-X-D-X-E, are remarkably conserved among all these chitinases except for chitodextrinase (EndoI) from V. furnissii (12). Therefore, to investigate the role of ChiD in the chitin degradation system of the strain, biochemical properties of the recombinant ChiD were examined. Bacterial chitinases generally yield (GlcNAc)2 as the major product of hydrolysis of chitin. Thus, we compared properties of ChiD with those of ChiA to elucidate the individual roles in the chitin degradation system. The cleavage patterns of both enzymes analyzed by TLC showed that ChiD hydrolyzed (GlcNAc)3 or longer oligosaccharides by an endo-type mechanism. However, ChiD was very slightly active toward chitin even after prolonged incubation. These results indicate that ChiD possesses unique structural and enzymatic properties distinguished from those of chitinases so far reported. ChiD showed properties similar to those of EndoI from V. furnissii (12), which is a solitary instance regardless of the increase of the number of chitinolytic enzymes. However, they differ from each other in the following points. (i) ChiD contains two chitin-binding domains. Therefore, ChiD adsorbs to α-chitin as shown in Fig. 6, but EndoI does not. (ii) Glutamic acid of the consensus sequence D-X-D-X-E is a catalytic amino acid residue of family 18 chitinases (38). The glutamic acid residue is found in the catalytic region of ChiD, but an arginine residue is substituted for the glutamic acid residue in EndoI. (iii) ChiD shows detectable activity with (GlcNAc)3, but EndoI does not. (iv) ChiD is a secreted protein (unpublished data), whereas EndoI is a periplasmic protein. These biochemical properties and the cellular location of ChiD suggest that its major role in the chitin degradation system is different from that of EndoI. The major role of ChiD in the chitin degradation system is to hydrolyze (GlcNAc)3 or longer oligosaccharides which are the intermediates of products of hydrolysis of chitin by chitinases. We are testing the synergistic effect of ChiD on the chitin degradation rate of ChiA, ChiB, and ChiC. Furthermore, the function of the N- and C-terminal ChtBDs of ChiD in the breakdown of chitin oligosaccharides and whether the ChtBD binds soluble chitin oligosaccharides from trimers to hexamers remain to be investigated.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 2.Brun, E., F. Moriaud, P. Gans, M. J. Blackledge, F. Barras, and D. Marion. 1997. Solution structure of the cellulose-binding domain of the endoglucanase Z secreted by Erwinia chrysanthemi. Biochemistry 36:16074–16086 [DOI] [PubMed] [Google Scholar]
- 3.Dall, D., A. Sriskantha, A. Vera, J. Lai-Fook, and T. Symonds. 1993. A gene encoding a highly expressed spindle body protein of Heliothis armigera entomopoxvirus. J. Gen. Virol. 74:1811–1818 [DOI] [PubMed] [Google Scholar]
- 4.Folders, J., J. Tommassen, L. C. van Loon, and W. Bitter. 2000. Identification of a chitin-binding protein secreted by Pseudomonas aeruginosa. J. Bacteriol. 182:1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garda, A. L., J. M. Fernandez-Abalos, P. Sanches, A. Ruiz-Arribas, and R. I. Santamaria. 1997. Two genes encoding an endoglucanase and a cellulose-binding protein are clustered and co-regulated by a TTA codon in Streptomyces halstedii JM8. Biochem. J. 324:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction from amino acid sequence data. Anal. Biochem. 182:319–326 [DOI] [PubMed] [Google Scholar]
- 7.Gooday, G. W. 1990. The ecology of chitin degradation. Adv. Microb. Ecol. 11:387–430 [Google Scholar]
- 8.Gross, C. H., G. M. Wolgamot, R. L. Russell, M. N. Pearson, and G. F. Rohrmann. 1993. A 37-kDa glycoprotein from a baculovirus of Orgyia pseudotsugata is localized to cytoplasmic inclusion bodies. J. Virol. 67:469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrissat, B., and A. Bairoch. 1996. Updating the sequence-based classification of glycosyl hydrolases. Biochem. J. 316:695–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyhani, N. O., and S. Roseman. 1996. The chitin catabolic cascade in the marine bacterium Vibrio furnissii. Molecular cloning, isolation, and characterization of periplasmic chitodextrinase. J. Biol. Chem. 271:33414–33424 [DOI] [PubMed] [Google Scholar]
- 13.Li, H., H. Plattner, K. L. Schimz, M. Kiess, H. Diekmann, and J. Meens. 2000. Cloning, sequencing and heterologous expression of a new chitinase gene, chi92, from Streptomyces olivaceoviridis ATCC11238. Biotechnol. Lett. 22:1203–1209 [Google Scholar]
- 14.Liu, J. J., and E. B. Carstens. 1996. Identification, molecular cloning, and transcription analysis of the Choristoneura fumiferana nuclear polyhedrosis virus spindle-like protein gene. Virology 223:396–400 [DOI] [PubMed] [Google Scholar]
- 15.Lonhienne, T., K. Mavromat, C. E. Vorgias, L. Buchon, C. Gerday, and V. Bouriotis. 2001. Cloning, sequencing, and characterization of two chitinase genes from the Antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J. Bacteriol. 183:1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsuhashi, W., Y. Furuta, and M. Sato. 1998. The spindles of an entomopoxvirus of coleoptera (Anomala cuprea) strongly enhance the infectivity of a nucleopolyhedrovirus in lepidoptera. J. Invertebr. Pathol. 71:186–188 [DOI] [PubMed] [Google Scholar]
- 17.Montgomery, M. T., and D. L. Kirchman. 1993. Role of chitin-binding proteins in the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl. Environ. Microbiol. 59:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morimoto, K., S. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 1999. Sequencing, expression, and transcription analysis of the Clostridium paraputrificum chiA gene encoding chitinase ChiA. Appl. Microbiol. Biotechnol. 51:340–347 [DOI] [PubMed] [Google Scholar]
- 19.Ohishi, K., K. Murase, T. Ohta, and H. Etoh. 2000. Cloning and sequencing of a chitinase gene from Vibrio alginolyticus H-8. J. Biosci. Bioeng. 89:501–505 [DOI] [PubMed] [Google Scholar]
- 20.Phanis, C. G., D. P. Miller, S. C. Cassar, M. Tristem, S. M. Thiem, and D. R. O’Reilly. 1999. Identification and expression of two baculovirus gp37 genes. J. Gen. Virol. 80:1823–1831 [DOI] [PubMed] [Google Scholar]
- 21.Pruzzo, C., A. Crippa, S. Bertone, L. Pane, and A. Carli. 1996. Attachment of Vibrio alginolyticus to chitin mediated by chitin-binding domain. Microbiology 142:2181–2186 [DOI] [PubMed] [Google Scholar]
- 22.Roffey, P. E., and J. M. Pemberton. 1990. Cloning and expression of an Aeromonas hydrophila chitinase gene in Escherichia coli. Curr. Microbiol. 21:329–337 [Google Scholar]
- 23.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y
- 24.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnellmann, J., A. Zeltins, H. Blaak, and H. Schrempf. 1994. The novel lectin-like protein CHB1 is encoded by a chitin-inducible Streptomyces olivaceoviridis gene and binds specifically to α-chitin of fungi and other organisms. Mol. Microbiol. 13:807–819 [DOI] [PubMed] [Google Scholar]
- 26.Sitrit, Y., C. E. Vorgias, I. Chet, and A. B. Oppenheim. 1995. Cloning and primary structure of the chiA gene from Aeromonas caviae. J. Bacteriol. 177:4187–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki, K., M. Suzuki, M. Taiyoji, N. Nikaidou, and T. Watanabe. 1998. Chitin binding protein (CBP21) in the culture supernatant of Serratia marcescens 2170. Biosci. Biotechnol. Biochem. 62:128–135 [DOI] [PubMed] [Google Scholar]
- 28.Tanaka, T., S. Fujiwara, S. Nishikori, T. Fukui, M. Takagi, and T. Imanaka. 1999. A unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1. Appl. Environ. Microbiol. 65:5338–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Techkarnjanaruk, S., and A. E. Goodman. 1999. Multiple genes involved in chitin degradation from the marine bacterium Pseudoalteromonas sp. strain S91. Microbiology 145:925–934 [DOI] [PubMed] [Google Scholar]
- 30.Tsujibo, H., Y. Yoshida, C. Imada, Y. Okami, and Y. Inamori. 1992. Isolation and characterization of a chitin degrading marine bacterium belonging to the genus Alteromonas. Fish. Sci. 57:2127–2131 [Google Scholar]
- 31.Tsujibo, H., H. Orikoshi, H. Tanno, K. Fujimoto, K. Miyamoto, C. Imada, Y. Okami, and Y. Inamori. 1993. Cloning, sequence, and expression of a chitinase gene from a marine bacterium, Alteromonas sp. strain O-7. J. Bacteriol. 175:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujibo, H., K. Fujimoto, H. Tanno, K. Miyamoto, C. Imada, Y. Okami, and Y. Inamori. 1994. Gene sequence, purification and characterization of N-acetyl-β-glucosaminidase from a marine bacterium, Alteromonas sp. strain O-7. Gene 146:111–115 [DOI] [PubMed] [Google Scholar]
- 33.Tsujibo, H., K. Fujimoto, H. Tanno, K. Miyamoto, Y. Kimura, C. Imada, Y. Okami, and Y. Inamori. 1995. Molecular cloning of the gene which encodes β-N-acetylglucosaminidase from a marine bacterium, Alteromonas sp. strain O-7. Appl. Environ. Microbiol. 61:804–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujibo, H., H. Orikoshi, K. Shiotani, M. Hayashi, J. Umeda, K. Miyamoto, C. Imada, Y. Okami, and Y. Inamori. 1998. Characterization of chitinase C from a marine bacterium, Alteromonas sp. strain O-7, and its corresponding gene and domain structure. Appl. Environ. Microbiol. 64:472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsujibo, H., J. Miyamoto, N. Kondo, K. Miyamoto, N. Baba, and Y. Inamori. 2000. Molecular cloning of the gene encoding an outer-membrane-associated β-N-acetylglucosaminidase involved in chitin degradation system of Alteromonas sp. strain O-7. Biosci. Biotechnol. Biochem. 64:2512–2516 [DOI] [PubMed] [Google Scholar]
- 36.Tsujibo, H., N. Kondo, K. Tanaka, K. Miyamoto, N. Baba, and Y. Inamori. 1999. Molecular analysis of the gene encoding a novel transglycosylative enzyme from Alteromonas sp. strain O-7 and its physiological role in the chitinolytic system. J. Bacteriol. 181:5461–5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Heijne, G. 1983. Patterns of amino acids near signal sequence cleavage sites. Eur. J. Biochem. 133:17–21 [DOI] [PubMed] [Google Scholar]
- 38.Watanabe, T., K. Kobori, K. Miyashita, T. Fujii, H. Sakai, M. Uchida, and H. Tanaka. 1993. Identification of glutamic acid 104 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J. Biol. Chem. 268:18567–18572 [PubMed] [Google Scholar]
- 39.Yu, C., A. M. Lee, B. L. Bassler, and S. Roseman. 1991. Chitin utilization by marine bacteria. J. Biol. Chem. 266:24260–24266. [PubMed] [Google Scholar]