Abstract
Occupational and environmental exposure to polycyclic aromatic hydrocarbons (PAHs) has been suggested to provoke inflammatory and/or allergic disorders, including asthma, rhinitis, and dermatitis. The molecular mechanisms of this PAH-mediated inflammation remain to be clarified. Previous studies implied the involvement of PAHs as irritants and allergens, with the reactive oxygen species generated from the oxygenated PAHs believed to be an exacerbating factor. It is also possible that PAHs contribute to the pathogenesis through activation of aryl-hydrocarbon receptor (AhR)-mediated transcription, since PAHs are potent inducers of the AhR. To address this point, we generated transgenic mouse lines expressing the constitutive active form of the AhR in keratinocytes. In these lines of mice, the AhR activity was constitutively enhanced in the absence of ligands, so that any other direct effects of PAHs and their metabolites could be ignored. At birth, these transgenic mice were normal, but severe skin lesions with itching developed postnatally. The skin lesions were accompanied by inflammation and immunological imbalance and resembled typical atopic dermatitis. We demonstrate that constitutive activation of the AhR pathway causes inflammatory skin lesions and suggests a new mechanism for the exacerbation of inflammatory diseases after exposure to occupational and environmental xenobiotics.
A steady increase in the prevalence of allergic diseases has been noted over the last century (30). Exposure to environmental xenobiotics was reported as one of the risk factors associated with the development of atopy and asthma (3). Polycyclic aromatic hydrocarbons (PAHs) are one of major environmental pollutants present in automobile exhaust, cigarette smoke, various foods, and industrial wastes. The skin, respiratory tract, and digestive tract are the first tissues that come into contact with these exogenous chemicals. Recent studies suggested that inhalation of PAHs in diesel exhaust particles and cigarette smoke triggers inflammatory responses in the respiratory tract, resulting in rhinitis and asthma (13, 21, 31, 33). Occupational exposure to PAHs or topical application of chemicals or drugs containing PAHs elicits inflammatory skin diseases, known as contact hypersensitivity or dermatitis (36, 37). In spite of the increasing number of reports showing a relationship between PAHs and inflammatory disorders, the precise molecular mechanisms by which such chemicals contribute to the development of pathological states remain to be clarified.
The carcinogenic and mutagenic effects of PAHs are well documented (for instance, see review in reference 32) and genetic and biochemical studies indicate that most of these responses elicited by PAHs are mediated through binding to aryl-hydrocarbon receptor (AhR) (22), since PAHs are potent inducers of the AhR activity (12). On the contrary, the involvement of AhR in the inflammatory effect of PAHs is controversial, since dioxins, a typical group of ligands for AhR, suppress the allergen-specific immune responses (10, 35) and often induce chloracne, whose clinical and histopathological appearance is rather distinct from those of PAH-mediated contact dermatitis (6, 37). Usually, the latter is accompanied by itching and is associated with inflammation, whereas the former does not display these signs even in the late stages of the disease (6, 37). Instead, the PAHs have been suggested to provoke inflammation as primary irritants or by allergic mechanisms against PAHs or their metabolites (1, 7, 37). Other lines of evidence suggest that reactive oxygen species generated by oxygenated PAHs appear to enhance the allergic response (4, 15). PAHs have also been shown to stimulate an increase in the DNA-binding activity of NF-κB, which in turn induces cytokine gene expression and provokes the allergic response (28).
The effect of environmental xenobiotics on the immune systems has been intensively examined, but uncertainty remains as to whether these compounds do indeed influence immune responses. Difference in the experimental systems seems to give rise to distinct results. For instance, there is a report showing that PAHs and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) directly enhance the immunoglobulin E (IgE) production when added to the purified B cells (33). Another study showed that TCDD preincubation results in the decrease of IgE production from the B-cell culture (14). This situation convinced us that an integrated in vivo experimental system should be established for evaluating and dissecting the biological effect of these compounds.
We surmised that, in any case, AhR-mediated transcription should be activated in the tissues where the AhR ligands are applied. In order to clarify the contribution of AhR-mediated transcription to the development of inflammatory disorders, we attempted to discriminate between the contribution of the AhR pathway and the other effects of PAHs. Therefore, in the present study, we assessed the direct contribution of the AhR pathway to inflammatory disorders by utilizing the constitutive active form of AhR (AhR-CA) that can activate transcription in the absence of exogenous chemicals. Skin was chosen as the tissue to examine AhR-CA expression because the consequences are easy to observe, and transgenic mice were generated that express AhR-CA in keratinocytes under the regulation of keratin 14 promoter (11). These transgenic mice were normal at birth, but severe skin lesions with itching developed postnatally. The skin lesions were accompanied by inflammation and immunological imbalance and resembled typical atopic dermatitis. This result clearly shows that constitutive activation of the AhR pathway is sufficient to trigger the inflammatory skin lesions and suggests the involvement of AhR-mediated transcription in the inflammatory diseases after exposure to occupational and environmental xenobiotics.
MATERIALS AND METHODS
Generating transgenic mice expressing AhR-CA.
Male and female BDF1 mice were purchased from CLEA Japan (Tokyo) to obtain fertilized eggs for DNA microinjection. AhR-CA transgenic mice, generated as described below, were maintained in the mixed background of C57BL/6 and DBA/2. AhR-CA transgenic mice and their wild-type littermates were housed in plastic cages in an air-conditioned room at a temperature of 24 ± 1°C, a humidity of 55% ± 5%, and a 12-h light-dark cycle.
For the construction of the pK14-AhR-CA-HA transgene, we first made a cDNA fragment encoding the constitutive active form of mouse AhR (AhR-CA) with a hemagglutinin (HA) tag sequence at its 3′ end (pBSK-AhR-CA-HA). The ligand-binding domain (277 to 418 amino acids [aa]) of AhR was deleted to generate AhR-CA. In brief, we generated AhR-CA cDNA by ligating the PCR-amplified fragments that encode the N-terminal (1 to 276 aa) and C-terminal (419 to 805 aa) halves of the AhR with an internal linker sequence (GGGGGATCGGG), and the resultant cDNA fragment was subcloned into pBluescript II SK(+) vector (pBSK-mAhR-CA). An HA tag sequence was added to the 3′ end to make pBSK-AhR-CA-HA. To construct pK14-AhR-CA-HA, the blunt-ended HindIII/XbaI fragment of the HA-tagged AhR-CA cDNA was subcloned into the blunt-ended BamHI and XbaI sites of a human K14 promoter/enhancer cassette plasmid (a generous gift from E. Fuchs [11]). The construct was linearized, purified, and injected into fertilized oocytes. The transgenic mice were identified by the PCR (ca. 30 to 32 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s) with a primer set: 5′-AAC TCT CTG TTC TTA GGC TCA GCG TC-3′ and 5′-ATA CGC TCT GAT GGA TGA CAT CAG ACT-3′. Fluorescence in situ hybridization (FISH) analysis was performed with the splenocytes prepared from Line 234 female mice and the pK14-AhR-CA-HA transgene as a probe through the standard procedure by Trans Animex Co., Ltd. (Hokkaido, Japan).
Immunoblotting analysis.
Dorsal skins were homogenized in buffer containing 20 mM HEPES (pH 7.6), 20% glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% protease inhibitor, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. Homogenized samples were mixed with an equal volume of gel loading buffer (100 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate, 0.2% bromophenol blue, 20% glycerol, 200 mM dithiothreitol) and then sonicated and boiled at 100°C for 3 min. Soluble fractions were collected as supernatants after centrifugation. An aliquot (40 μg) of each sample was separated by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis and transferred onto Immobilon-P Transfer Membrane (Millipore Corp., Bedford, MA). The membrane was incubated in anti-HA antibody (sc-805; Santa Cruz Biotechnology, CA). The signal was detected using ECL and a Western blotting detection system (Amersham Pharmacia Biotech).
RNA blotting analysis.
Total RNA was prepared from dorsal skin by using Isogen (Nippon Gene, Tokyo) according to the manufacturer's instructions. Total RNA (15 μg) was separated by electrophoresis on a 1% formaldehyde-agarose gel and subsequently transferred onto Zeta-Probe GT Genomic Tested Blotting Membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was probed with 32P-labeled CYP1A1 cDNA fragment.
Histological and immunohistochemical analyses.
Skins were fixed in 3.7% formaldehyde, embedded in paraffin, and sectioned. For histological analysis, the samples were stained with hematoxylin and eosin. Alcian blue staining was used for detecting mast cell granules. For the detection of CYP1A1, samples were allowed to react with anti-CYP1A1 antibody (sc-9828; Santa Cruz Biotechnology) overnight. Anti-CYP1A1 antibody binding was detected with horseradish peroxidase-conjugated anti-goat IgG antibody and diaminobenzidene was used as a chromogen.
Measurement of grooming duration and scratching frequency.
Individual mice were kept in a cage in a calm state and monitored by video camera for 30 min. Grooming duration and scratching frequency were measured over a randomly selected 10-min period. Grooming of any part of the body using the forepaw or mouth was taken as a grooming episode, and a tally was made of the duration of each episode within the 10-min period. A series of scratching behavior using the hind paw was taken as one scratching episode, and the frequency of the episodes were counted for 10 min. Three mice were examined independently at 6 weeks of age.
cDNA microarray analysis.
Ten-day-old AhR-CA mice of Line 239 (two males and three females) and their wild-type littermates (two males and two females) were sacrificed, and total RNAs were isolated from their back skin. The isolated RNAs were further purified by RNeasy RNA isolation kit (QIAGEN). Purified RNA was used for preparing labeled cRNA that was hybridized to a mouse expression array 430A gene chip (Affymetrix, Santa Clara, CA). Experimental procedures for GeneChip were performed according to the Affymetrix GeneChip expression analysis technical manual. The results were deposited in the Gene Expression Omnibus (GEO) at the National Center for Biotechnology Information (GEO accession number GSE2955).
cDNA synthesis and reverse transcription-PCR (RT-PCR).
Total RNA (3 μg) was reverse transcribed by Superscript II (Invitrogen) with 150 ng of random hexamer at 42°C. Each cDNA was amplified by PCR using ExTaq (TaKaRa Shuzo, Tokyo, Japan). The primers used were 5′-AGT GCA GAC AGT CCA CAG CA-3′ and 5′-TGC TCA GAG TAG TGA CCG AAC G-3′ for CYP1B1, 5′-TCA GTT CCC ATT GCA GTG G-3′ and 5′-TGG AAT GGA CTT GCC C-3′ for NQO1, 5′-ACA CGG TGC TGG ACG AAT C-3′ and 5′-ACG TAC GCC CAG TGA A-3′ for ADH, 5′-TTA CCA TGG GAG CTG AG-3′ and 5′-CCA GAG CGA AGC CAT TGA G-3′ for AhRR, 5′-ACC CGG ACC CAA AAC TTA G-3′ and 5′-TGT TTG CCA GCA GTG ATC-3′ for keratin 1, 5′-TTG AGC AGT ACA TCA GCA A-3′ and 5′-GGA TCA TGC GGT TGA-3′ for keratin 6, 5′-TCA TTA TTG CCA CCC AGG A-3′ and 5′-TCT CCA GGC CCT GGA A-3′ for keratin 16, 5′-TTC CTT GCT TTG GCA TG-3′ and 5′-CAG TTC TGC TTT GGA TC-3′ for CCL20, 5′-CAA CTT TGG CCG ACT TC-3′ and 5′-GAGT GAA CAT TAC AGA TTT ATC CCC-3′ for IL-18, 5′-CTA TCG TGC TCG AAT GAA CAC-3′ and 5′-GCC AAC AGG AAG CTG AGA GT-3′ for HO-1, and 5′-CGA GCA CAG CTT CTT T-3′ and 5′-AGG TAG TCT GTC AGG T-3′ for β-actin.
Measurement of serological parameters.
Thirteen AhR-CA mice and twelve wild-type littermates aged 7 to 14 weeks were examined. Blood was drawn from retro-orbital vessels. IgE, IgG1, IgG2a, IgG3, and IgM were quantified by using enzyme-linked immunosorbent assay (ELISA). Cytokine production from spleen T cells stimulated with anti-CD3 monoclonal antibody. We treated 48-well flat-bottom microplates with 2 μg of anti-CD3 antibody (145-2C11; BD Pharmingen) and 0.5 μg of anti-CD28 antibody (PV-1; SBA). Splenocytes were prepared from seven AhR-CA mice and eight wild-type littermates aged from 7 to 14 weeks. A total of 5 × 106 cells were seeded per well and cultured with 1 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum for 24 h. The culture supernatants were then collected and interleukin-2 (IL-2), IL-4, IL-5, IL-12, and gamma interferon (IFN-γ) were quantified with ELISA.
RESULTS
Generation of transgenic mouse lines expressing AhR-CA in keratinocytes and integration of transgene into X chromosome in one of the lines.
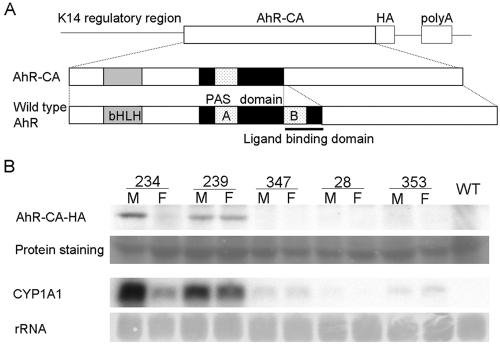
In order to explore the possibility that activation of the AhR pathway can elicit inflammatory response in vivo, we generated transgenic lines of mice that express AhR-CA in keratinocytes under the regulation of the keratin 14 (K14) promoter (Fig. 1A). Among several lines of mice generated, two (Lines 234 and 239) were found to express the AhR-CA transgene at high levels (Fig. 1B). Since the expression of AhR-CA in female Line 234 mice was consistently weaker than that in male mice of Line 234, we mapped the chromosomal localization of the transgene by using FISH analysis. The result unveiled that the transgene was integrated into the X chromosome in Line 234 (data not shown). Thus, we suspected that the transgene was subjected to random inactivation of the X chromosome (i.e., lionization [29]) and that the female skin was composed of two kinds of keratinocytes, the cells expressing the transgene and the cells whose transgenes were silenced, consequently resulting in the lowered expression of AhR-CA in female mice compared to male mice when the whole skin was examined.
FIG. 1.
Generation of AhR-CA transgenic mice. (A) Structure of the transgene. Basic helix-loop-helix (bHLH) and PAS domains are indicated as shaded and dotted boxes, respectively. The PAS-B domain serving as a ligand-binding domain (indicated by a bar) was deleted to generate the AhR-CA molecule. An HA tag was added at the C terminus of the protein. (B) Expression of the transgene correlates with the CYP1A1 mRNA level. HA-tagged AhR-CA was detected by immunoblot analysis with anti-HA antibody. CYP1A1 mRNA was detected by RNA blot analysis. The corresponding line numbers are shown at the top. M, male; F, female; WT, wild type.
Since the male mice of Line 234 died before weaning (data not shown), an essential gene on the X chromosome must have been disrupted by integration of the transgene. Line 234 mice, especially female mice, were found to provide strong evidence for a link between activation of the AhR pathway and the development of inflammatory lesions as will be described below. The abundance of AhR-CA showed a good correlation with the abundance of CYP1A1 mRNA, one of the target gene products of the AhR (Fig. 1B), demonstrating that AhR-mediated transcription is activated in the skin of the transgenic mice without any exogenous stimuli.
AhR-CA transgenic mice postnatally develop skin lesions with itching.
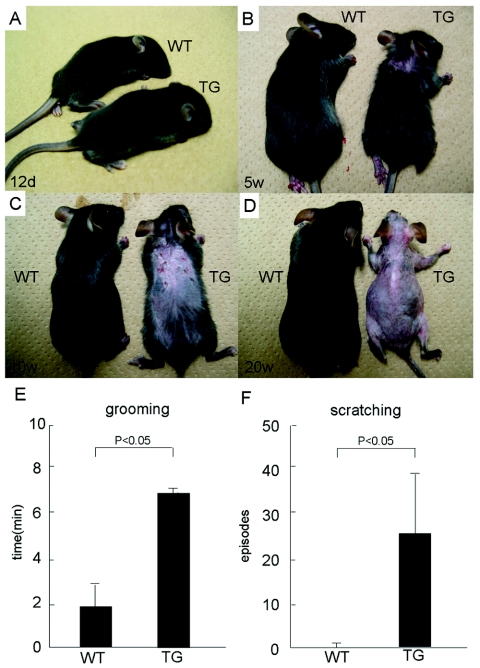
Whereas Line 239 mice were normal at birth and healthy up to 12 days of age (Fig. 2A), they gradually developed erosive eczema in the back skin by 3 to 5 weeks after birth (Fig. 2B). Eventually, a massive loss of their fur coats occurred after the tenth week, even under specific-pathogen-free conditions (Fig. 2C and D). The whole skin over the body was subjected to the same process of skin lesion development. We noticed that both female and male mice of Line 239 frequently scratched their skin, which started around the time of weaning. Closer examination with the video tape recording revealed that both male and female transgenic mice of Line 239 performed grooming for longer periods of time and scratched much more frequently than their wild-type littermates (Fig. 2E and F). An important observation was that, although the transgene was already expressed in the embryos of the late fetal stage (data not shown), skin disorders accompanied by itching and scratching developed postnatally in Line 239 mice, suggesting that postnatal epidermal development and/or mechanical stimuli may be required in addition for completion of the skin lesion pathogenesis.
FIG. 2.
Macroscopic observation of Line 239 mice. (A) Twelfth day after birth. Note that skin lesions are not apparent. (B) Fifth week. An eczematous change emerges on the back skin. (C) Tenth week. Erosive dermatitis with crusts is observed. (D) Twentieth week. Note that re-epithelialization follows, and the fur coat is completely lost. (E and F) Observation of behaviors related to skin itching. The total duration of grooming behavior within 10 min was measured for three independent mice, and the averages and standard deviations are shown in panel E. The frequency of scratching episodes within 10 min was counted for three independent mice, and the averages and standard deviations are shown in panel F. The differences between the AhR-CA mice (TG) and the wild-type control mice (WT) are statistically significant (P < 0.05).
Epidermal and dermal pathological changes observed in AhR-CA transgenic mice.
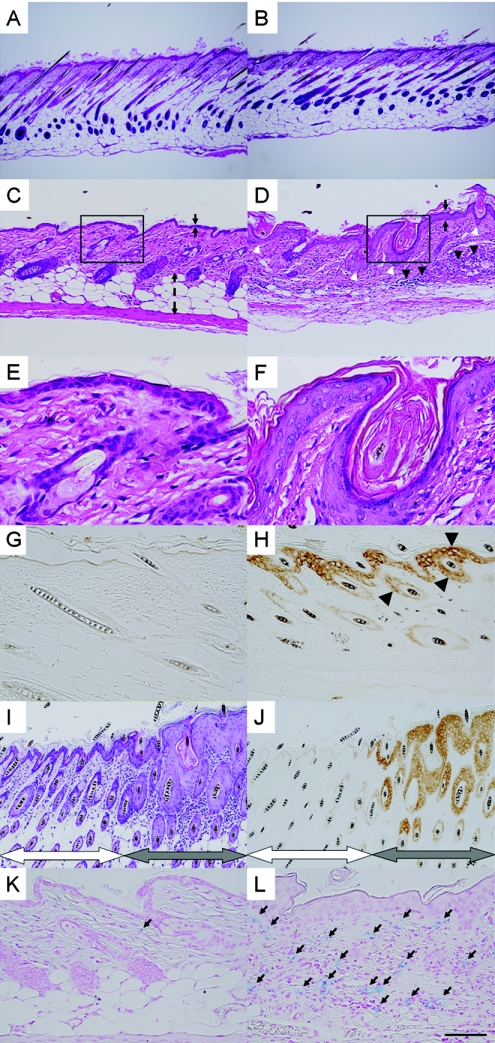
To examine the skin of symptomatic Line 239 mice in more detail, histological analysis was performed. Consistent with the macroscopic observation, there was no apparent abnormality during the first week after birth (Fig. 3A and B). However, prominent acanthosis and hyperkeratinization developed by 4 weeks of age (Fig. 3D and F); most hair follicles were dilated and filled with layers of keratinized cells (6, 37). The dermis was severely infiltrated with lymphocytes and polymorphonuclear cells, and the subcutaneous fat tissue had mostly disappeared (Fig. 3D and F).
FIG. 3.
Histological examination of the dorsal skin of AhR-CA mice. (A to F) Skin specimens from Line 239 AhR-CA transgenic mice (B, D, and F) and from wild-type mice (A, C, and E) were stained with hematoxylin and eosin. The samples were prepared at the age of 1 week (A and B) or 4 weeks (C to F). The black arrows (C and D) indicate the widths of the epidermal layers, and the dashed double arrowheads (C) indicate the width of the subcutaneous fat tissue. Black arrowheads (D) indicate cell infiltration, and white arrowheads (D) indicate dilated hair follicles filled with keratinized cell masses. Boxed areas in panels C and D are shown at a higher magnification in panels E and F, respectively. (G and H) Immunohistochemical detection of CYP1A1 expression. A 10-day-old Line 239 mouse (H) and its wild-type littermate (G) were examined. Intense brown signals can be seen in the epidermal and follicular keratinocytes (arrowheads) of the transgenic mouse, whereas no apparent signals can be detected in the control sample. (I and J) Serial sections prepared from the skin of a Line 234 adult female mouse. One was stained with hematoxylin and eosin (I), and the other was exposed to anti-CYP1A1 antibody (J). The left-hand halves of the micrographs (indicated by white double arrowheads) show the normal state of the skin, while the right-hand halves (indicated by gray double arrowheads) show inflammatory changes. (K and L) Alcian blue staining of the skin specimens from the 4-week- old mice of Line 239 (L) and wild-type littermate (K). Black arrows (K and L) indicate mast cells that have infiltrated the dermis. Scale bar: 40 μm (A and B), 16 μm (C, D, K, and L), 8 μm (G to J), 4 μm (E and F).
Although the macroscopic skin lesions observed in Line 234 were milder than in Line 239, both lines shared similar histopathological changes. Male Line 234 mice, with the highest transgene expression (see Fig. 1B), developed the pathological state by 2 weeks of age, which was slightly earlier than the onset of Line 239 mice (data not shown). Further exacerbation could not be observed in male Line 234 mice since the mice die before weaning due to a probable insertion mutagenesis or disruption of an essential gene on the X chromosome by the transgene. Intriguingly, adult female Line 234 mice displayed chimeric patterns in their skin lesions, which made the dermatitis macroscopically less apparent. Both normal and inflammatory parts resided side by side (Fig. 3I), and this pattern was quite consistent with that of the target gene expression (Fig. 3J). This observation may be best explained by lionization of the transgene integrated into X chromosome. We can assume that, in the normal part of skin, the AhR-CA transgene was inactivated due to the lionization of the transgene-harboring X chromosome, whereas, in the inflammatory part of the skin, the expression of the transgene persisted due to the transgene-harboring X chromosome escaping from the lionization. None of our low AhR-CA transgene expressor lines showed any such skin abnormalities (data not shown). These results thus indicate that the severity of the skin disorders correlates well with the expression levels of the AhR-CA transgene.
We then monitored the activity of AhR-CA protein in the skin through detecting expression of its target gene, CYP1A1. When CYP1A1 expression in the transgenic mice was examined immunohistochemically, intense signals were detected specifically in epidermal keratinocytes, an observation consistent in Line 239 mice (Fig. 3H) and male Line 234 mice at 10 days after birth (data not shown). As expected, female Line 234 mice displayed a chimeric staining pattern (Fig. 3J). A patch of epidermis with intense CYP1A1 expression displayed acanthotic proliferation and hyperkeratinization, and the underlying dermis was infiltrated with inflammatory cells (Fig. 3I and J). However, such skin alterations were not observed in the portions without a detectable CYP1A1 expression. These results clearly indicate that the AhR pathway is activated specifically in keratinocytes and that this keratinocyte-specific AhR activation causes the observed inflammatory skin disorders.
Alcian blue staining of the skin sections revealed that a large number of mast cells had accumulated in the dermis in Line 239 (Fig. 3L). This was also evident in Line 234 males at 10 days after birth (data not shown) but less evident in Line 234 females. Since mast cells are considered to be a critical factor inducing skin itching, we can deduce that the skin lesions are intimately associated with the itching.
Inflammation-related genes are increased in AhR-CA mice.
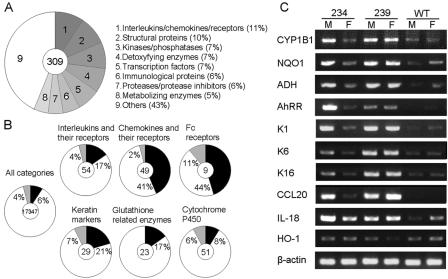
To further clarify the molecular basis of the skin lesions, we performed a DNA microarray analysis with skin RNA samples from 10-day-old Line 239 mice, an age representing initiation of the skin disorders (Table 1 and Fig. 4). This early stage was chosen because we were curious about how the skin lesions were initiated. Total RNAs from five Line 239 mice and four wild-type littermates were pooled as AhR-CA RNA and wild-type RNA, respectively, and these two samples were compared. Among 309 genes that were increased >4-fold in the transgenic mice compared to wild-type mice, 7% were involved in detoxification, while, surprisingly, nearly a quarter were inflammation-related genes, including interleukins/chemokines and their receptors, immunological proteins, and proteases and their inhibitors (Fig. 4A). We looked at genes with an expression either upregulated or downregulated by at least twofold and found that the ratios of the upregulated genes were higher in the categories of the inflammation-related genes (Fig. 4B).
TABLE 1.
Genes increased in the skin of AhR-CA mice
| Gene category | Gene subcategory | Accession no. | Gene | Fold change |
|---|---|---|---|---|
| Detoxifying enzymes | Detoxifying enzymes and genes | NM009644 | Aryl-hydrocarbon receptor repressor | 16.6 |
| NM009992 | Cytochrome P450, 1a1 | 44.2 | ||
| NM009994 | Cytochrome P450, 1b1 | 4.0 | ||
| NM007825 | Cytochrome P450, 7b1 | 3.7 | ||
| AV158882 | NAD(P)H dehydrogenase, quinone 1 | 3.8 | ||
| NM010357 | Glutathione S-transferase, alpha 4 | 8.2 | ||
| NM008184 | Glutathione S-transferase, mu 6 | 2.0 | ||
| NM008161 | Glutathione peroxidase 3 | 2.7 | ||
| Interleukins, chemokines, and receptors | Interleukins and their receptors | AF000304 | IL-4 receptor, alpha | 15.1 |
| L20048 | IL-2 receptor, gamma chain | 8.0 | ||
| D13695 | IL-1 beta | 6.0 | ||
| NM008360 | IL-18 | 2.2 | ||
| Chemokines | BC002073 | Chemokine (C-C motif) ligand 6 | 18.5 | |
| NM009141 | Chemokine (C-X-C motif) ligand 5 | 22.2 | ||
| AF099052 | Chemokine (C-C motif) ligand 20 | 14.9 | ||
| NM008176 | Chemokine (C-X-C motif) ligand 1 | 8.9 | ||
| NM011888 | Chemokine (C-C motif) ligand 19 | 8.4 | ||
| NM021443 | Chemokine (C-C motif) ligand 8 | 6.3 | ||
| Immunological proteins | Fc receptors | NM010185 | Fc receptor, IgE, high-affinity I, Gamma polypeptide | 23.9 |
| BM224327 | Fc receptor, IgG, low-affinity IIb | 6.8 | ||
| Antimicrobial peptide | NM019728 | Defensin beta 4 | 38.3 | |
| NM013756 | Defensin beta 3 | 11.0 | ||
| Structure proteins | Keratins | NM010669 | Keratin 6b | 35.1 |
| NM008470 | Keratin 16 | 7.8 | ||
| NM008473 | Keratin 1 | 6.1 | ||
| Proteases and inhibitors | Proteases | NM008572 | Mast cell protease 8 | 11.1 |
| NM008571 | Mast cell protease 2 | 2.8 | ||
| NM008607 | Matrix metalloproteinase 13 | 34.8 | ||
| AV375008 | Matrix metalloproteinase 19 | 9.1 | ||
| NM013599 | Matrix metalloproteinase 9 | 3.3 |
FIG. 4.
Altered gene expression profiles in the skin of AhR-CA mice. (A) A total of 309 genes increased by >4-fold are categorized into nine groups. The percentage of each category is shown. (B) The ratios of the genes increased (black areas) or deceased (gray areas) by >2-fold are shown within each gene subcategory. The number written in the center of each circle graph indicates the number of genes included in that subcategory. (C) The expression levels of representative genes were examined by semiquantitative RT-PCR. cDNAs were synthesized from the skin RNAs of 10-day-old AhR-CA and wild-type mice. The corresponding line numbers are shown at the top. M, male; F, female; WT, wild type.
We then selected representative genes from the microarray analysis and examined their expression levels by RT-PCR analysis with 10-day-old skin RNAs (Fig. 4C). All of the genes, except for heme oxygenase-1 (HO-1), were consistently increased in Line 239 and male Line 234 mice and slightly increased in female Line 234 mice. For instance, the detoxifying enzymes CYP1B1, NAD(P)H:quinone oxidoreductase 1, alcohol dehydrogenase, and AhR repressor were higher in the transgenic mice compared to wild-type levels. As for the inflammation-related genes, keratins 1, 6, and 16 (K1, K6, and K16), CCL20, and IL-18 were dramatically increased in Line 239 and male Line 234 mice, reflecting the proinflammatory state of the skin of these transgenic mice.
We found that IL-18 and CCL20 were also highly expressed in the fetal skin of AhR-CA mice soon after the start of transgene expression (data not shown). In addition, considering that the upstream regions of the IL-18 and CCL20 genes contain multiple AhR binding sites (xenobiotic response elements [XRE]), IL-18 and CCL20 could be the primary genes activated by AhR-CA in keratinocytes to trigger the inflammatory responses.
An intriguing observation was that, HO-1, considered a sensitive marker of the oxidative stress generated by exposure to PAHs (20), was not induced in the AhR-CA transgenic mice, suggesting that contribution of reactive oxygen species to the development of the skin lesions might be marginal.
The cell-mediated immune response is predominant in AhR-CA transgenic mice.
We then examined characteristics of fully developed skin inflammation observed in AhR-CA transgenic mice using adult Line 239 mice. Since local inflammation is often accompanied by systemic immune imbalance, we assessed whether the AhR-CA transgenic mice display any signs of disturbance of the immune system, particularly, imbalance between Th1 and Th2 cells. Differentiated CD4+ lymphocytes (helper T cell) residing in spleen are divided into two populations, Th1 cells and Th2 cells, based on the potential ability of cytokine production. IFN-γ and IL-2 are the cytokines preferentially produced by Th1 cells, and IL-4 and IL-5 are the ones produced by Th2 cells when they are stimulated (e.g., by anti-CD3 antibody).
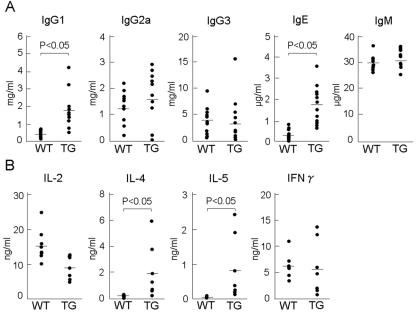
To examine the balance between Th1 and Th2 cell populations, we first measured the serum concentration of immunoglobulin and found that the concentrations of both IgG1 and IgE in the sera of Line 239 mice were markedly elevated compared to those in the wild-type littermates (Fig. 5A). Since the production of IgG1 and IgE is promoted by the cytokines derived from Th2 lymphocytes, it was strongly suggested that a Th2 cell-mediated immune response was predominant in symptomatic mice and that systemic immune balance was secondarily affected by epidermal proinflammatory responses.
FIG. 5.
Spontaneous deviation of splenic lymphocytes into Th2 cells. (A) High serum levels of IgE and IgG1 in AhR-CA mice. Sera from the AhR-CA mice (n = 13) and their wild-type littermates (n = 12) aged from 7 to 14 weeks were sampled. The serum levels of various types of immunoglobulins (IgG2a, IgG3, IgM, IgE, and IgG1) were measured by ELISA. Differences in the levels of IgE and IgG1 are statistically significant between the transgenic mice and controls (P < 0.05). (B) Increased production of IL-4 and IL-5 from the anti-CD3 stimulated splenic lymphocytes. Splenic lymphocytes were prepared from the AhR-CA mice (n = 7) and their wild-type littermates (n = 8) aged from 7 to 14 weeks and then stimulated with anti-CD3 antibody. The cytokine concentrations (IL-4, IL-5, IFN-γ, and IL-2) in each supernatant were determined by ELISA. Differences in the levels of IL-4 and IL-5 are statistically significant between the transgenic mice and their controls (P < 0.05).
To further address this point, we then isolated and cultured splenocytes of adult Line 239 mice and measured the cytokine production. Consistent with our current results, splenocytes from the symptomatic mice produced much more IL-4 and IL-5, which were secreted from Th2 cells, in response to immobilized anti-CD3 antibody (Fig. 5B). On the other hand, the production of IL-2 and IFN-γ (secreted by Th1 cells) was lower in the Line 239 mice than in wild-type mice. Taken together, we concluded that overexpression of AhR-CA gives rise to high serum IgE and IgG1 levels and a dominant Th2 response. It is noteworthy that these immune responses are often common background phenotypes accompanying atopic dermatitis (19) and a frequent consequence of exposure to environmental xenobiotics, including diesel exhaust particles (8).
DISCUSSION
We demonstrated here that keratinocyte-targeted overexpression of the AhR-CA protein represents an effective way of generating skin lesions that mimic atopic dermatitis and PAH contact hypersensitivity (8, 19). We successfully generated inflammatory skin lesions with no systemic or topical application of any exogenous chemicals, but by purely activating AhR-mediated transcription in epidermal keratinocytes. Our microarray result showed that many inflammation-related genes were actually upregulated, which is a good reflection of our unique strategy. These results clearly indicate that activation of the AhR signaling pathway itself is sufficient to initiate the inflammatory disorders.
The K14 promoter was reported to be active in the whole layers of epidermal keratinocytes, including follicular keratinocytes, when examined in transgenic mice (11). This expression pattern was consistent with that of CYP1A1 examined here (see Fig. 3H) and similar to that of endogenous AhR in skin (J. Mimura and Y. Fujii-Kuriyama, unpublished observations). Thus, AhR-CA expression driven by the K14 promoter was not merely ectopic but rather mimicked endogenous AhR distribution, allowing an interpretation that AhR-CA mice reflect a condition where AhR ligands are applied to the skin without operating AhR-independent action.
Transgenic mice expressing AhR-CA under the regulation of the mouse immunoglobulin heavy-chain enhancer showed an elevated risk in the spontaneous development of stomach tumors (2) and chemical hepatocarcinogenesis (23). When AhR-CA was expressed in mice under the regulation of T cell-specific CD2 promoter, the number of thymocytes was decreased and immunization-induced T-cell or B-cell expansion was suppressed (26). Importantly, none of these reports described the development of inflammation in any tissues. In contrast, the present results provide convincing evidence that the local activation of the AhR is critical for provoking the inflammatory responses in the tissues involved. The patchy skin lesions observed in Line 234 females nicely support this contention. Thus, avoidance of local exposure to PAHs should be effective in preventing inflammatory disorders mediated by PAHs.
The development of inflammatory skin diseases has been suggested to be the result of interactions between the immune system and skin-derived molecules (18, 25). Indeed, it was previously suggested that topically applied PAHs exert a systemic influence on immune organs (16). Although the precise mechanism remains to be elucidated, we speculate that this either occurs as a direct effect of PAHs on the immune cells after percutaneous absorption or by the secondary activation of immune organs following proinflammatory responses of the skin. The AhR-CA transgenic mice displayed skin lesions accompanied by the systemic immune imbalance shifted toward the Th2 cell predominance, which could be the secondary effect of the epidermal proinflammatory responses mediated by AhR-CA protein. In addition, the direct activation of the AhR pathway in the immune organs also seemed to be responsible for the full completion of the pathological state of the transgenic mice. Consistent with the previous observation of K14 promoter activity (9), we found the additional expression of AhR-CA in the thymus (data not shown), which may have contributed to the altered immune system together with cytokines and chemokines derived from the skin lesions.
There is an interesting discrepancy between the onset of apparent inflammation in histology and that of inflammation-related gene induction. The former coincides with the postnatal period around the weaning age, and the latter starts within the late fetal stage in utero. Although the precise reason why the skin lesions develop only after birth remains unknown, we speculate that maturation of the immune systems may be required before the primary triggering signals originated from the keratinocytes come into effect for the progression of the inflammatory process. The development and maturation of skin itself might also be required.
Scratching behavior seems to exacerbate the progression of the skin lesions, ending in the massive loss of fur coats. Although prominent acanthosis and hyperkeratinization with dermal infiltration of lymphocytes and polymorphonuclear cells were commonly observed in both Line 239 and Line 234 mice, the severe scratching and dramatic skin lesions were prominently observed in Line 239 mice but not in Line 234 mice. The male mice of Line 234 die around the weaning, so they do not live until they were able to scratch their skin. Female mice of Line 234 scratch their skin only mildly; probably the itching may not be so strong because of the spotted inflammation in their skin. Hence, the further evolvement of the skin lesions seems to correlate well with the presence of scratching behavior.
One important question remained is which genes are responsible for triggering the skin inflammation in response to the constitutively active AhR. Among the various genes induced in the transgenic skin, IL-18 and CCL20 are good candidates for triggering the inflammatory responses. IL-18 is known to induce inflammatory skin diseases similar to atopic dermatitis when expressed in mice through the K14 promoter (17). Similarly, if secreted from keratinocytes, CCL20 would be able to trigger skin inflammation by directing the migration of inflammatory cells (34). Indeed, there has been a strong suggestion of a correlation between CCL20 and atopic dermatitis (24). Multiple XRE found in the upstream regions of the IL-18 and CCL20 genes imply that these genomic regions are responsive to the activated AhR. However, other proinflammatory genes that do not contain an XRE in the proximal promoter region may also have contributed, since recent studies revealed the XRE-independent AhR function as a coactivator for other transcription factors (27). Further investigation is necessary for elucidation of the primary target genes that are activated by AhR-CA and trigger the inflammatory responses.
Intriguingly, the phenotype of the skin lesions, with remarkable cell infiltration and itching, resembles the contact dermatitis triggered by other PAHs (5) more than the chloracne triggered by dioxins (6, 37), implying the operation of distinct mechanisms underlying the two pathological conditions. A previous report showed that binding sequences are different between dioxin-liganded AhR and PAH-liganded AhR (21a), suggesting that a profile of gene activation by AhR after dioxin exposure could be different from that by AhR after PAH exposure. We surmise that a profile of gene activation by AhR-CA might be more similar to that by PAH-liganded AhR. Tissue delivery and distribution of dioxins and PAHs might be another factor to generate the phenotypic difference. It seems likely that AhR activation triggered in PAH contact dermatitis predominantly occurs in keratinocytes, as seen in AhR-CA mice we generated in the present study.
The present study demonstrates for the first time the primary contribution of AhR-mediated transcriptional activation to the development of inflammatory diseases. Since no exogenous chemical was required to trigger the inflammatory responses in this model system, activation of the downstream genes under AhR regulation is most likely to be responsible. Our findings suggest that the induction of AhR target genes is one of the central mechanisms of PAH-mediated inflammatory diseases. Based on these findings, the possibility emerges that blocking AhR signals may help to relieve the allergic symptoms, since xenobiotic exposure exacerbates allergic diseases through the AhR pathway.
Acknowledgments
We thank Yuko Kikuchi, Naomi Kaneko, and Reiko Kawai for assistance. We also thank Yoichi Matsuda and Kazuhiko Yamada at Trans Animex for FISH analysis, Tania O'Connor for help with editing the manuscript, and Shogo Yamamoto for help in deposition of the microarray data.
This study was supported by grants from ERATO-JST (M.Y.); the Ministry of Education, Science, Sports, and Culture (H.M. and M.Y.); the Ministry of Health, Labor, and Welfare (M.Y.); the Atherosclerosis Foundation (M.Y.); SORST-JST (Y.F.-K. and H.M.); the Yamanouchi Foundation for Research on Metabolic Disorders (H.M.); the Uehara Memorial Foundation (H.M.); and the Special Coordination Fund for Promoting Science and Technology (H.M.). F.K. is a JSPS Research Fellow.
REFERENCES
- 1.Anderson, C., A. Hehr, R. Robbins, R. Hasan, M. Athar, H. Mukhtar, and C. A. Elmets. 1995. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J. Immunol. 155:3530-3537. [PubMed] [Google Scholar]
- 2.Andersson, P., J. McGuire, C. Rubio, K. Gradin, M. L. Whitelaw, S. Pettersson, A. Hanberg, and L. Poellinger. 2002. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. USA 99:9990-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arruda, L. K., D. Sole, C. E. Baena-Cagnani, and C. K. Naspitz. 2005. Risk factors for asthma and atopy. Curr. Opin. Allergy Clin. Immunol. 5:153-159. [DOI] [PubMed] [Google Scholar]
- 4.Bonvallot, V., A. Baeza-Squiban, A. Baulig, S. Brulant, S. Boland, F. Muzeau, R. Barouki, and F. Marano. 2001. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am. J. Respir. Cell. Mol. Biol. 25:515-521. [DOI] [PubMed] [Google Scholar]
- 5.Casale, G. P., Z. Cheng, J. Liu, E. L. Cavalieri, and M. Singhal. 2000. Profiles of cytokine mRNAs in the skin and lymph nodes of SENCAR mice treated epicutaneously with dibenzo[a,l]pyrene or dimethylbenz[a]anthracene reveal a direct correlation between carcinogen-induced contact hypersensitivity and epidermal hyperplasia. Mol. Carcinog. 27:125-140. [DOI] [PubMed] [Google Scholar]
- 6.Coenraads, P. J., A. Brouwer, K. Olie, and N. Tang. 1994. Chloracne: some recent issues. Dermatol. Clin. 12:569-576. [PubMed] [Google Scholar]
- 7.Davila, D. P. D. R. Davis, K. Campbell, J. C. Cambier, L. A. Zigmond, and S. W. Burchiel. 1995. Role of alterations in Ca2+-associated signaling pathways in the immunotoxicity of polycyclic aromatic hydrocarbons. J. Toxicol. Environ. Health 45:101-126. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Sanchez, D. 1997. The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy 52:52-56. [DOI] [PubMed] [Google Scholar]
- 9.Frazer, I. H., G. J. P. Fernando, N. Fowler, G. R. Leggatt, P. F. Lambert, A. Liem, K. Malcolm, and R. W. Tindle. 1998. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur. J. Immunol. 28: 2791-2800. [DOI] [PubMed] [Google Scholar]
- 10.Fujimaki, H., K. Nohara, T. Kobayashi, K. Suzuki, K. Eguchi-Kasai, S. Tsukumo, M. Kijima, and C. Tohyama. 2002. Effect of a single oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin on immune function in male NC/Nga mice. Toxicol. Sci. 66:117-124. [DOI] [PubMed] [Google Scholar]
- 11.Guo, L., Q. C. Yu, and E. Fuchs. 1993. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 12:973-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankinson, O. 1995. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35:307-340. [DOI] [PubMed] [Google Scholar]
- 13.Heo, Y., A. Saxon, and O. Hankinson. 2001. Effect of diesel exhaust particles and their components on the allergen-specific IgE and IgG1 response in mice. Toxicology 159:143-158. [DOI] [PubMed] [Google Scholar]
- 14.Karras, J. G., D. H. Conrad, and M. P. Holsapple. 1995. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on interleukin-4-mediated mechanisms of immunity. Toxicol. Lett. 75:225-233. [DOI] [PubMed] [Google Scholar]
- 15.Kepley, C. L., F. T. Lauer, J. M. Oliver, and S. W. Burchiel. 2003. Environmental polycyclic aromatic hydrocarbons, benzo(a)pyrene(BaP) and BaP-quinones, enhance IgE-mediated histamine release and IL-4 production in human basophils. Clin. Immunol. 107:10-19. [DOI] [PubMed] [Google Scholar]
- 16.Klemme, J. C., H. Mukhtar, and C. A. Elmets. 1987. Induction of contact hypersensitivity to dimethylbenz(a)anthracene and benzo(a)pyrene in C3H/HeN mice. Cancer Res. 47:6074-6078. [PubMed] [Google Scholar]
- 17.Konishi, H., H. Tsutsui, T. Murakami, S. Yumikura-Futatsugi, K. Yamanaka, M. Tanaka, Y. Iwakura, N. Suzuki, K. Takeda, S. Akira, K. Nakanishi, and H. Mizutani. 2002. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proc. Natl. Acad. Sci. USA 99:11340-11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung, D. Y. 1999. Pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 104:99-108. [DOI] [PubMed] [Google Scholar]
- 19.Leung, D. Y., and T. Bieber. 2003. Atopic dermatitis. Lancet 361:151-160. [DOI] [PubMed] [Google Scholar]
- 20.Li, N., A. C. Sioutas, Cho, D. Schmitz, C. Misra, J. Sempf, M. Wang, T. Oberley, J. Froines, and A. Nel. 2003. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 111:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastrangelo, G., C. Veller Fornasa, S. Pavanello, G. Mercer, M. Lazzaro, Milan, E. G. Fadda, U. Fedeli, and E. Clonfero. 2003. Polyaromatic hydrocarbons administered in humans by dermal route increase total IgE. Int. J. Immunopathol. Pharmacol. 16:145-150. [DOI] [PubMed] [Google Scholar]
- 21a.Matikainen, T., G. I. Perez, A. Jurisicova, J. K. Pru, J. J. Schlezinger, H. Y. Ryu, J. Laine, T. Sakai, S. J. Korsmeyer, R. F. Casper, D. H. Sherr, and J. L. Tilly. 2001. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 28:355-360. [DOI] [PubMed] [Google Scholar]
- 22.Mimura, J., K. Yamashita, K. Nakamura, M. Morita, T. N. Takagi, K. Nakao, M. Ema, K. Sogawa, M. Yasuda, M. Katsuki, and Y. Fujii-Kuriyama. 1997. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2:645-654. [DOI] [PubMed] [Google Scholar]
- 23.Moennikes, O., S. Loeppen, A. Buchmann, P. Andersson, C. Ittrich, L. Poellinger, and Schwarz. M. 2004. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 64:4707-4710. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama, T., R. Fujisawa, H. Yamada, T. Horikawa, H. Kawasaki, K. Hieshima, D. Izawa, S. Fujiie, T. Tezuka, and O. Yoshie. 2001. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3α/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 13:95-103. [DOI] [PubMed] [Google Scholar]
- 25.Nickoloff, B. J. 1999. Skin innate immune system in psoriasis: friend or foe. J. Clin. Investig. 104:1161-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohara, X. K., Pan, S. Tsukumo, A. Hida, T. Ito, H. Nagai, K. Inouye, H. Motohashi, M. Yamamoto, Y. Fujii-Kuriyama, and C. Tohyama. 2005. Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization- induced increase in splenocytes. J. Immunol. 174:2770-2777. [DOI] [PubMed] [Google Scholar]
- 27.Ohtake, F., K. Takeyama, T. Matsumoto, H. Kitagawa, Y. Yamamoto, K. Nohara, C. Tohyama, A. Krust, J. Mimura, P. Chambon, J. Yanagisawa, Y. Fujii-Kuriyama, and S. Kato. 2003. Modulation of estrogen receptor signalling by association with the activated dioxin receptor. Nature 423:545-550. [DOI] [PubMed] [Google Scholar]
- 28.Pei, X. H., Y. Nakanishi, H. Inoue, K. Takayama, F. Bai, and N. Hara. 2002. Polycyclic aromatic hydrocarbons induce IL-8 expression through nuclear factor κB activation in A549 cell line. Cytokine 19:236-241. [PubMed] [Google Scholar]
- 29.Pereira, L. V., and L. R. Vasques. 2000. X-chromosome inactivation: lessons from transgenic mice. Gene 255:363-371. [DOI] [PubMed] [Google Scholar]
- 30.Rydzynski, K., and C. Palczynski. 2004. Occupational allergy as a challenge to developing countries. Toxicology 198:75-82. [DOI] [PubMed] [Google Scholar]
- 31.Saxon, A., and D. Diaz-Sanchez. 2000. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology 48:325-327. [DOI] [PubMed] [Google Scholar]
- 32.Sjogren, M., L. Ehrenberg, and U. Rannug. 1996. Relevance of different biological assays in assessing initiating and promoting properties of polycyclic aromatic hydrocarbons with respect to carcinogenic potency. Mutat. Res. 358:97-112. [DOI] [PubMed] [Google Scholar]
- 33.Takenaka, H., K. Zhang, D. Diaz-Sanchez, A. Tsien, and A. Saxon. 1995. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J. Allergy Clin. Immunol. 95:103-115. [DOI] [PubMed] [Google Scholar]
- 34.Tohyama, M., Y. Shirakara, K. Yamasaki, K. Sayama, and K. Hashimoto. 2001. Differentiated keratinocytes are responsible for TNF-alpha regulated production of macrophage inflammatory protein 3α/CCL20, a potent chemokine for Langerhans cells. J. Dermatol. Sci. 27:130-139. [DOI] [PubMed] [Google Scholar]
- 35.Walker, D. B., W. C. Williams, C. B. Copeland, and R. J. Smialowicz. 2004. Persistent suppression of contact hypersensitivity, and altered T-cell parameters in F344 rats exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicology 197:57-66. [DOI] [PubMed] [Google Scholar]
- 36.Wu, M. T., C. H. Pan, T. N. Wu, Y. L. Huang, C. Y. Chen, L. H. Huang, and C. K. Ho. 2003. Immunological findings in a group of coke-oven workers exposed to polycyclic aromatic hydrocarbons. J. Occup. Environ. Med. 45:1034-1039. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto, O., and Y. Tokura. 2003. Photocontact dermatitis and chloracne: two major occupational and environmental skin diseases induced by different actions of halogenated chemicals. J. Dermatol. Sci. 32:85-94. [DOI] [PubMed] [Google Scholar]