Abstract
Adaptive immunity is crucial for protective host defense and the development of immunological disorders. SLY1 was recently identified as an X-chromosomal SH3 protein that is serine phosphorylated (Ser27) upon B-and T-cell receptor engagement. Here, we demonstrate that SLY1 is localized in the cytoplasm and the nucleus of immunocytes. We generated mice expressing a mutant version of SLY1 lacking Ser27 and a functional nuclear localization signal. The defective SLY1 (SLY1d) protein is localized exclusively in the cytoplasm. B- and T-cell proliferation is attenuated and T-cell cytokine production is severely reduced. Sly1d/d mice exhibit reduced lymphoid organ sizes, diminished marginal zone B-cell numbers, and severely impaired antibody responses against T-dependent and -independent antigens. Importantly, survival of semi-identical cardiac allografts was substantially prolonged in Sly1d/d mice. These results define SLY1 as an essential molecular component for the full activation of adaptive immunity.
Immune reactions are triggered by various stimuli including conserved microbial components that activate the innate immune system and cognate antigens that activate T and B lymphocytes in adaptive immunity. CD4 T lymphocytes can differentiate in distinct functional classes that differ in the secretion of effector cytokines and play central regulatory roles in adaptive immune responses. The lineage decisions for differentiation of helper T cells may be influenced by cytokine receptor signaling as well as signals derived from costimulatory receptors (21). TH1 cells produce gamma interferon and are responsible for host defense through cell-mediated immunity. Under pathological conditions, TH1 cells may promote autoimmune reactions as well as rejection of allografts. TH2 cells secrete interleukin- (IL-)4 as their major effector cytokine and support humoral immune responses, but may also be involved in autoimmune disorders (21, 37). Recently primed CD4 T helper cells migrate to the T-cell zone of B-cell follicles where they encounter antigen-specific B cells that have also migrated to this area (9). CD4 T cells deliver, primarily through CD40, a signal for B-cell expansion and antibody isotype switching (7,26, 38).
Both B and T lymphocytes use antigen receptor engagement to initiate distinct signal transduction pathways that direct ultimate cellular responses. It is well established that adaptor molecules regulate the coupling of receptor-proximal events, such as protein tyrosine kinase activation, with results such as inducible gene expression and cytoskeletal rearrangements. While adaptor molecules possess no intrinsic enzymatic activity, their ability to mediate protein-protein interactions is vital for the integration and propagation of signal transduction cascades in lymphocytes (12, 16, 29). Adaptor molecules may contain multiple phosphorylatable residues or proline-rich regions that allow them to be recognized by interaction domains such as SH2 and SH3 on other signaling proteins. Therefore, adaptor molecules are able to coordinate interactions among the signal transduction effector proteins. This characteristic suggests that adaptor molecules act as intracellular scaffolds around which signaling complexes are organized. The use of genetically manipulated mice has shown that adaptor proteins play essential roles in T and B lymphocyte development and in activation of adaptive immune responses (31).
Recently, we have identified the putative adaptor protein SLY1 (SH3 domain protein expressed in lymphocytes), which contains a bipartite nuclear localization signal as well as SH3 and SAM domains. It is preferentially expressed in T and Blymphocytes (4). Using a phosphopeptide-specific antiserum it was demonstrated that SLY1 is phosphorylated on Ser27 following T- or B-cell receptor ligation (2). Moreover, inhibitor studies implied that SLY1 represents a target for phosphorylation by phosphatidylinositol 3-kinase, protein kinase C, or a protein kinase C-dependent protein kinase in antigen receptor-stimulated lymphocytes. These studies therefore defined SLY1 as a previously unrecognized target for antigen receptor signal transduction and suggested that it may play a role in adaptive immunity.
In the present study, Sly1 mutant mice were generated expressing a SLY1 protein that lacks the Ser27 phosphorylation site and parts of the nuclear localization signal (SLY1Δaa 20-100). In contrast to the wild-type SLY1 protein, the mutant SLY1 protein is not localized in the nucleus. Sly1d/d mice reveal impaired lymphoid organ development and antigen receptor-mediated lymphocyte activation. Sly1 mutant mice exhibit severe defects in humoral immune responses. Finally, Sly1d/d mice show extended allograft survival. Thus, expression of intact SLY1 protein appears crucial for adaptive immune responses.
MATERIALS AND METHODS
Reagents.
All antibodies used in flow cytometry analyses and in vitro B- and T-cell stimulation experiments were obtained from BD Pharmingen except for anti-immunoglobulin M F(ab′)2, which was purchased from Jackson ImmunoResearch. For Western blot analyses, antibodies against mitogen-activated protein kinases and IκBα were obtained from Cell Signaling, whereas antiphosphotyrosine antibody 4G10 was from Upstate Signaling. Rabbit polyclonal antisera against SLY1 were produced against peptides LRRKPSNASDKEPTQ (anti-SLY1 residues 2 to 16) and TEQEEREPPSL-SRQT (anti-SLY1 residues 131to 145) according to standard protocols. Recombinant IL-2 and recombinant IL-4 were from R&D Systems, and lipopolysaccharide was from Sigma. Concentrations of IL-2, IL-4, IL-10, tumor necrosis factor, and gamma interferon in culture supernatants were measured using enzyme-linked immunosorbent assay kits from R&D Systems. Trinitrophenol (TNP)-CGG, TNP-Ficoll, and TNP-bovine serum albumin were from Biosearch Technologies, and alum was obtained from Serva. phorbol myristate acetate, ionomycin, Na3Ov4 and protease inhibitory cocktail were purchased from Sigma.
Gene targeting and mice.
E14.1 embryonic stem (ES) cells from 129/Ola micewere grown in Dulbecco's modified Eagle's medium (GIBCO BRL) supplemented with l-glutamine (2 mM; Seromed), leukemia inhibitory factor, penicillin/streptomycin (100 μg/ml; Seromed), 2-mercaptoethanol (0.05 mM; GIBCO BRL), and 15% heat-inactivated fetal bovine serum (Boehringer).
A murine genomic ES-129 bacterial artificial chromosome library (Genome Systems Inc.) was screened by hybridization with a 205-bp murine Sly1 cDNA fragment as a probe. The resulting bacterial artificial chromosome clone was mapped by Southern blot hybridization using murine Sly1 cDNA fragments. bacterial artificial chromosome fragments were cloned into pBluescript (Stratagene) and fully sequenced. The targeting vector was constructed in pBluescript such that a 600-bp fragment of the genomic Sly1 locus encoding exon 2 and 3 was replaced by lacZ and a neomycin resistance cassette. A herpes simplex virus thymidine kinase cassette was inserted 3 kb upstream of the targeted sequence. E14.1 ES cells were electroporated with the NotI-linearized targeting vector, and the transfected cells subjected to G418 and ganciclovir selection. Clones showing homologous recombination were detected by PCR and subsequently confirmed by Southern blot hybridization with the 3′ flanking probe indicated in Fig. 1a after digestion of ES cell DNA with EcoRI. Single integration was verified by probing the Southern blot with the neomycin resistance cassette. Correctly targeted ES cell clones were injected into C57BL/6 blastocysts, which were transferred into pseudopregnant foster mothers. Resulting chimeric mice were backcrossed to C57BL/6 mice, and germ line transmission of the targeted allele was confirmed by Southern blot analysis.
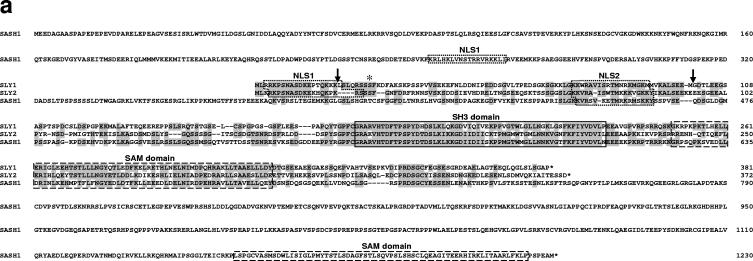
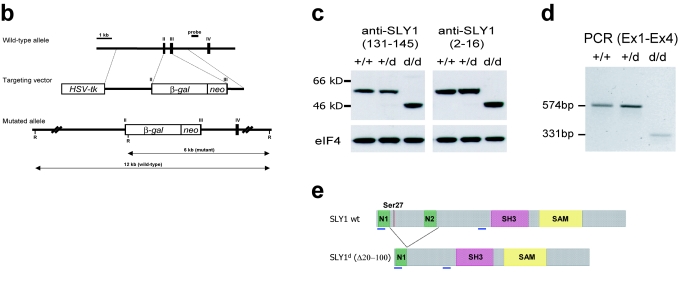
FIG. 1.
Generation of Sly1 mutant mice. (a) Amino acid sequence alignment of murine SLY1, SLY2, and SASH1 proteins is shown. Identical residues are shaded and protein domains are indicated by boxes. The arrows assign the deletion in SLY1d caused by skipping of exons 2 and 3. The asterisk marks phosphorylated Ser27. (b) The organization of part of the murine Sly1 genomic locus (top), the linearized targeting vector (middle), and the targeted Sly1 allele (bottom) are shown. The β-galactosidase cDNA and the neomycin resistance cassette were inserted between codon 26 (exon 2) and codon 72 (exon 3) of the Sly1 gene. The β-galactosidase cDNA contains a polyadenylation signal. Exon sequences are represented by solid rectangles, intron sequences by a black line (R, EcoRI). (c) Lysates of splenocytes from wild-type (+/+), heterozygous (+/d), and homozygous mutant (d/d) mice were analyzed by Western blot with two independent rabbit polyclonal antisera directed against amino acids 2 to 16 or 131 to 145 of the wild-type SLY1 protein. Antisera specific for eIF4 served as loading control. (d) The skipping of exons 2 and 3 was revealed by reverse transcription-PCR with exon 1- and exon 4-specific primers in splenic mRNA from wild-type (+/+) and heterozygous (+/d) as well as homozygous (d/d) mutant mice. (e) Schematic representation of the mutant SLY1d protein. N, nuclear localization signal; blue lines, immunogenic peptides for generation of SLY1-specific antisera.
Mice were kept according to national guidelines for animal care in an SPF animal facility. The mice used in these experiments were backcrossed to the C57BL/6 background for 6 to 8 generations. Wild-type littermates were used as controls. Genotyping for the Sly1 mutation was performed by PCR with the following primers: 5′-GATCCATGCCAGCGTTACCA-3′(WT/forward), 5′-TCGCCTTCTATCGCCTTCTTG(Neo/for), and 5′-AGTCATAGCTCTCCATCAGC-3′(reverse).
For reverse transcription-PCR analysis of SLY1 mRNA splicing, total RNA from thymus and spleen was extracted using the TRIZOL reagent (Invitrogen Life Technologies), and was reverse-transcribed to cDNA using oligo(dT) primer and SuperScript II (Invitrogen Life Technologies). The following primer set was used for exon skipping analysis: Ex1_for: 5′-GAGTAGCAGCCCCAGGC-3′, and Ex4_rev: 5′-CTGTTGATGTCTGGCGGC-3′.
Generation of bone marrow chimeric mice.
For generation of bone marrow chimeric mice, recipients were lethally irradiated with 6 Gy and intravenously injected with 107 donor bone marrow cells 24 h later. Analysis of mice was performed 4 weeks after adoptive bone marrow transfer.
B-cell proliferation.
Splenocytes from wild-type and Sly1d/d mice were depleted of non-B cells using immunomagnetic beads conjugated with antibodies against CD90, CD11b, and CD11c (Miltenyi Biotech). Enriched B cells were stained with CD23 and CD21 antibodies and marginal zone (CD21hi CD23lo) and follicular B cells (CD21int CD23hi) were purified on a cell sorter (MoFlo; DakoCytomation). Purified B-cell subsets were stimulated with antibodies against immunoglobulin M and CD40 (10 μg/ml each) for 3 days. [3H]thymidine (Amersham Pharmacia Biotech) was added after 56 h of culture and incubated for 16 h.
T-cell proliferation and cytokine production.
Splenocytes were incubated at 2× 105 cells per well in 96-well round-bottomed plates with the indicated soluble antibodies or reagents in 200 μl medium. For proliferation assays, 1 μCi [3H]thymidine (Amersham Pharmacia Biotech) was added after 66 h of culture and incubated for 16 h. Incorporation of [3H]thymidine was measured with a Matrix 96 direct γ counter (Packard Instrument Co.). For analysis of cytokine production, supernatants were harvested after 18 h of stimulation and assayed by enzyme-linked immunosorbent assay (all R&D Systems).
Allogeneic mixed lymphocyte reaction.
For induction of mixed lymphocyte reactions, allogeneic splenocytes (H-2d) were used as stimulator cells and cocultured with responder cells at concentrations of 2 × 106 cells/ml. The stimulator population was irradiated with 30Gy prior to coculture. The responder cells were total splenocytes (1.2 × 106 cells/ml), CD4-depleted splenocytes (1.5 × 106 cells/ml), or CD8-depleted splenocytes (1.7 × 106 cells/ml). Cell depletion was performed using anti-CD4 or anti-CD8 microbeads according to the manufacturer's instructions (Miltenyi Biotec). Depletion efficiency as determined by flow cytometry analysis was >99%. Proliferation was measured after 84 h of MLR activation by addition of 1 μCi/well 3[H]thymidine (Amersham Pharmacia Biotec) during the last 10 h of culture.
Cytotoxic lymphocyte assay.
Mice were immunized s.c. into the hind footpad with ovalbumin (150 μg/footpad; ovalbumin grade IV; Sigma) using CpG-DNA 1668 (5 nmol/footpad) as an adjuvant. Draining lymph nodes were removed 4 days later and cells were cultured for an additional 4 days in medium supplemented with 10 U/ml recombinant IL-2, which allows expansion of in vivo primed cytotoxic T lymphocytes precursors. 51Cr release assays were performed withEL4 target cells pulsed with 0.1 μM of the ovalbumin-derived peptide SIINFEKL. Serial dilutions of effector cytotoxic T lymphocytes were added to target cells and 51Cr release was measure after 4 h of coculture. Specific lysis was calculated according to the following formula: % specific lysis = [cpm (sample) - cpm (spontaneous release)/cpm (maximal release - cpm (spontaneous release)] × 100. For control, unpulsed EL4 cells were used as targets. Specific lysis of unpulsed EL4 cells was below 2% in all experiments.
Humoral immune responses.
Mice were immunized intraperitoneally with 5μg TNP-CGG (NP19-CGG) adsorbed to alum or with 100 μg TNP-Ficoll in phosphate-buffered saline. Sera were collected before and 4, 7, 14 and 21 days after immunization. Total NP-specific immunoglobulin isotypes (IgM, IgG1, IgG2a, and IgG3) were detected employing an enzyme-linked immunosorbent assay with NP14-bovine serum albumin-conjugated plates (10 μg/ml in carbonate-buffer pH 9.5). Sera were diluted 1:1,000 for TNP-CGG/alum and 1:500 for TNP-Ficoll immunizations. Murine NP-specific antibodies were detected with alkaline phosphatase-conjugated isotype-specific detection antibodies, which were obtained from Pharmingen (anti-IgG1, anti-IgG2a, and anti-IgG3) or Jackson ImmunoResearch (anti-IgM).
Allograft transplantations.
Vascularized cardiac grafts from donor mice were collected in anesthesia by dividing and excising the aorta and pulmonary artery as described (17). Donor hearts were placed in Brettschneider's solution (HTKKöhler Chemie, Germany) until grafting. Abdominal aorta and vena cava were prepared in recipient mice. Subsequently, the cardiac grafts were heterotypically transplanted by end-to-side anastomosis of donor aorta and pulmonary artery to the host's aorta and vena cava, respectively. Resumption of cardiac function of donor hearts was verified. Graft function was monitored daily by transabdominal palpation and scored. Rejection was defined as complete cessation of palpable heart beats and confirmed by direct inspection after laparotomy.
Signal transduction and subcellular protein localization.
To prepare total cell lysate, CD90+ T cells were incubated with biotinylated anti-CD3 antibody and cross-linked with streptavidin. Stimulation was stopped with ice-cold PBS and cells were subsequently treated with lysis buffer (150 mM NaCl, 50 mM Tris, pH 8, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% NP-40, 1 mM NaF, 2 mM Na3Ov4, and protease inhibitors) for 15 min on ice, and centrifuged at 13,000rpm for 10 min. The supernatants were supplemented with 5× Laemmli sample buffer, boiled for 5 min, and used for experiments. Nuclear and cytosolic fractions of cells were prepared according to standard procedures and the cytosolic fractions were precipitated with 10× volume acetone. Protein samples were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (4 to 12% gradient gel; Invitrogen) under reducing conditions, transferred to nitrocellulose membrane (Schleicher and Schuell), probed with the indicated primary antibodies, followed by reacting with horseradish peroxidase-conjugated secondary antibody, and developed by the ECL detection system (Amersham).
Ca influx analysis.
For Ca2+ flux analysis, splenocytes were loaded with 2.5μM Fluo-4 (Molecular Probes) for 25 min at 37°C and washed with RPMI 1640 (without phenol red) containing 25 mM HEPES. Cells were stained on ice with anti-CD8α-allophycocyanin (53.6.7; eBiosciences), anti-CD4-phycoerythrin-Cy5.5 (RM4 to 5; Caltag) and biotinylated anti-CD3ɛ. Cells were warmed to 37°C and analyzed for 20 seconds to establish baseline Ca2+ levels. Then, CD3ɛ was cross-linked by the addition of 10 μg/ml streptavidin (Sigma). The flow cytometric analysis was performed with a FACSCalibur (BD Biosciences) using FlowJo Software (TreeStar).
Gel shift analysis.
For electrophoretic mobility shift assays, nuclear extracts of CD90+ purified T cells were prepared according to standard procedures and the binding reaction was performed in the presence of a 32P-labeled oligonucleotide containing the specific recognition sites. Oligonucleotidess were from Santa Cruz: AP-1 (sc-2501), NF-AT (sc-3577), NF-κB (sc-2505), and Oct-1 (sc-2506).
RESULTS
Generation of a Sly1 mutant mouse strain.
The protein sequence analysis depicted in Fig. 1a demonstrates that SLY1 exhibits extensive homology to SLY2 (previously termed Nash1, HACS1, or SAMSN1) and SASH1 proteins (6, 35, 39). SLY1, SLY2, and SASH1 show a similar domain organization and consist of a putative bipartite nuclear localization sequence at the N terminus of the proteins followed by closely spaced SH3 and SAM domains. Compared to SLY1 and SLY2, SASH1 contains unique N- and C-terminal sequence extensions including a second SAM domain. Data base searches showed that the adjacent SH3 and SAM domains of SLY1, SLY2, and SASH1 are substantially more homologous to each other than to SH3 and SAM domains of other proteins suggesting that they define separate subfamilies of these protein modules (data not shown). Interestingly, the second SAM domain of SASH1 that is located at the C terminus of the protein is less well conserved. Due to their extensive sequence homology and unique domain organization SLY1, SLY2, and SASH1 constitute a new family of adaptor proteins.
Although SLY1, SLY2, and SASH1 are all highly expressed in hematopoietic cells, only the expression of SLY1 appears restricted to the lymphoid lineage (4, 6, 24, 35, 39). SLY1 has been shown to be phosphorylated in a PI3-kinase- or protein kinase C-dependent fashion upon B- and T-cell receptor engagement (2), but the role of SLY1 for the generation of immune responses is not known so far. To investigate the physiological functions of SLY1 in vivo, we replaced the majority of coding sequences of exons 2 and 3 of the Sly1 gene by homologous recombination in embryonic stem cells and generated homozygous mutant mice (Fig. 1b). Western blot analyses with two independent antisera raised against the N-terminal (amino acids 2 to 16) and central regions (amino acids 131 to 145) of the SLY1 protein revealed that homozygous mutant mice express a truncated protein of 45 kDa as opposed to the 55-kDa wild-type SLY1 protein (Fig. 1c).
Based on these findings we examined the possibility that the residual SLY1 protein was generated by a splicing event involving exons 1 and 4, thereby skipping the inserted β-galactosidase and neomycin resistance elements. Reverse transcription-PCR analyses using primers located in exons 1 and 4 detected a 574-bp fragment in wild-type mice and a 331-bp fragment in homozygous Sly1 mutant mice, which is consistent with an mRNA lacking exons 2 and 3 (Fig. 1d). Nucleotide sequence analysis of the 331-bp fragment directly demonstrated that it was derived from an in-frame splicing of exon 1to exon 4 of the mutant Sly1 allele (data not shown). Similar results were obtained when primers located in exons 1 and 5 were used for reverse transcription-PCR (data not shown). We conclude that the mutant Sly1 allele (hereafter designated Sly1d) encodes a truncated protein with a deletion encompassing amino acids Leu20 to Met100 (Fig. 1a, arrows). It should be noted that this deletion encompasses crucial structural elements of the SLY1 protein such as Ser27, which is phosphorylated upon antigen receptor engagement (2), and the C-terminal half of the putative bipartite nuclear localization sequence, rendering it functionally inactive (see below).
Altered lymphoid organogenesis and lymphocyte development in Sly1d/d mice.
Homozygous Sly1 mutant mice were born at normal Mendelian ratios, were fertile, and did not exhibit any gross anatomical or behavioral abnormalities (data not shown). Analysis of different lymphoid organs revealed that total cell numbers in thymus, spleen, peripheral lymph nodes, and Peyer's patches were significantly reduced by about 33 to 60% in Sly1d/d mice compared to wild-type littermates, whereas cellularity of bone marrow and peritoneal cavity appeared to be normal (Fig. 2a). In contrast to peripheral lymph nodes, the Sly1 mutation did not affect cell numbers in mesenteric lymph nodes. These data therefore extend previous work showing that organogenesis of peripheral and mesenteric lymph nodes requires distinct developmental triggers (1, 15, 25). In addition to reduced cellularity, the number of macroscopically visible Peyer's patches was significantly lower in Sly1d/d mice than in wild-type littermate controls (Fig. 2b). When examined by immunohistochemistry, lymphoid organs of Sly1d/d mice exhibited a normal distribution of B and T lymphocytes as well as nonlymphoid cells such as macrophages and dendritic cells (data not shown).
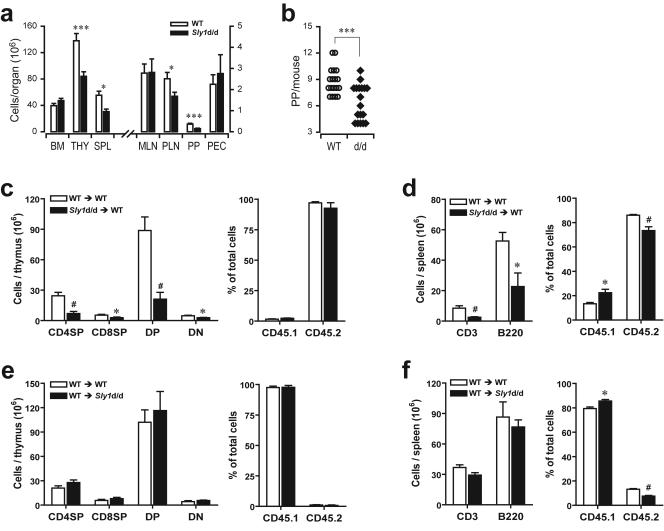
FIG. 2.
Influence of the Sly1d/d mutation on lymphoid organogenesis. (a) Total cell numbers were determined from bone marrow (BM), thymus (THY), spleen (SPL), mesenteric (MLN) and peripheral (PLN) lymph nodes, Peyer's patches (PP), and in peritoneal lavage (PEC) of wild-type littermates (white bars) and Sly1 mutant mice (solid bars) with 18 mice analyzed in each group. (b) Numbers of macroscopically visible Peyer's patches were counted. Each symbol represents an individual mouse (WT, wild-type littermate controls; d/d, Sly1 mutant mice). (c and e) Chimeric mice were generated by adoptive transfer of bone marrow cells from wild-type or Sly1d/d mice (both CD45.2+) into lethally irradiated CD45.1+ wild-type recipients or (d and f) by injection of CD45.1+ wild-type bone marrow cells into CD45.2+ wild-type and Sly1d/d recipients. Recipient mice were analyzed 4 weeks after adoptive transfer for CD4/CD8-defined thymic subsets (c and e) and the presence of CD3+ and B220+ cells in spleen (d and f). Results of adoptive transfer experiments are derived from 4 or 5 mice per group. *, P < 0.05; #, P < 0.01; ***, P < 0.001 (Student's t test). DP, double-positive; DN, double-negative.
The effects of the Sly1 mutation on lymphoid organ development may result from a function of SLY1 in lymphoid cells, in lymphoid organ stroma, or both. To address this question bone marrow chimeric mice were generated. In a first set of experiments, Sly1d/d or wild-type bone marrow cells (both CD45.2+) were injected into lethally irradiated congenic hosts (CD45.1+). The results in Fig. 2c demonstrate that the absolute cell numbers of all thymocyte subsets (defined by CD4 and CD8 expression) were significantly lower upon reconstitution with Sly1d/d compared to wild-type bone marrow cells, whereas the relative proportions of CD45.2+ donor-derived thymocytes were similar for both genotypes. In contrast, adoptive transfer of wild-type bone marrow cells (CD45.1+) reconstituted thymi in Sly1d/d and wild-type hosts with similar efficiency (Fig. 2e). Similarly, reconstitution of splenic B and T lymphocytes was impaired upon transfer of Sly1d/d bone marrow cells, whereas transfer of wild-type bone marrow cells into Sly1d/d hosts resulted in efficient spleen repopulation with B and T cells (Fig.2d and f). These results suggest that the Sly1d/d mutation impairs the ability of hematopoietic cells to reconstitute T and B-cell compartments rather than affecting lymphoid organ stroma.
To further characterize the effects of the Sly1d/d mutation on the development of T and B cells we performed flow cytometry analyses of various lymphoid organs. The results depicted in Table 1 and Fig. 3a show that Sly1d/d mice had significantly lower absolute numbers of CD4 and CD8 double-positive and CD4 single positive thymocytes than wild-type controls. Therefore, experiments were performed to examine the influence of the Sly1d/d mutation on thymocyte differentiation in more detail.
TABLE 1.
T- and B-cell development in Sly1d/d mutant micea
| Organ | Subset | Mean % positive cells ± SD
|
Mean no. of cells/organ (106) ± SD
|
||||
|---|---|---|---|---|---|---|---|
| Wild type | Sly1d/d | P | Wild type | Sly1d/d | P | ||
| Thymus | CD4− CD8− | 3.6 ± 1.4 | 6.2 ± 1.2 | <0.001 | 5.1 ± 1.8 | 5.7 ± 1.8 | 0.46 |
| CD4+ CD8+ | 81.6 ± 2.8 | 79.1 ± 2.7 | <0.05 | 110.5 ± 25.9 | 59.9 ± 14.5 | <0.001 | |
| CD4+ CD8− | 10.8 ± 1.4 | 9.0 ± 1.8 | <0.01 | 18.7 ± 5.2 | 8.7 ± 3.9 | <0.001 | |
| CD8+ CD4− | 3.5 ± 0.9 | 4.0 ± 1.1 | 0.21 | 5.4 ± 1.8 | 3.9 ± 1.2 | 0.10 | |
| Spleenb | CD4+ | 61.0 ± 1.8 | 49.2 ± 3.0 | <0.001 | 14.2 ± 4.4 | 7.1 ± 1.5 | <0.01 |
| CD8+ | 32.9 ± 1.5 | 39.7 ± 2.4 | <0.001 | 6.9 ± 1.2 | 5.7 ± 1.1 | 0.11 | |
| CD25+ | 2.0 ± 0.2 | 2.0 ± 0.1 | 0.86 | 1.2 ± 0.6 | 0.7 ± 0.2 | 0.20 | |
| IgMhi IgDlo | 6.2 ± 2.1 | 2.7 ± 1.0 | <0.01 | 3.5 ± 0.9 | 1.3 ± 0.4 | <0.01 | |
| IgMlo IgDhi | 32.3 ± 8.6 | 29.1 ± 11.8 | 0.62 | 23.9 ± 4.1 | 15.2 ± 9.8 | 0.15 | |
| Bone marrow | B220lo IgM− | 29.9 ± 6.0 | 25.0 ± 6.4 | 0.29 | 9.4 ± 1.4 | 10.0 ± 2.2 | 0.69 |
| B220lo IgMlo | 11.3 ± 6.0 | 13.8 ± 7.3 | 0.61 | 3.7 ± 1.7 | 6.2 ± 2.8 | 0.32 | |
| B220hi IgMhi | 7.0 ± 2.5 | 4.9 ± 2.5 | 0.26 | 2.0 ± 0.7 | 1.9 ± 1.0 | 0.16 | |
| Peritoneal cavity | IgMhi IgDlo | 16.0 ± 2.3 | 17.2 ± 5.0 | 0.79 | n.d. | n.d. | n.d. |
Data are means and are derived from 6 to 12 independent mice per group.
Percentages of CD4+ and CD8+ cells are relative to CD3+ cells, whereas percentages of CD25+ cells are relative to CD4 T cells.
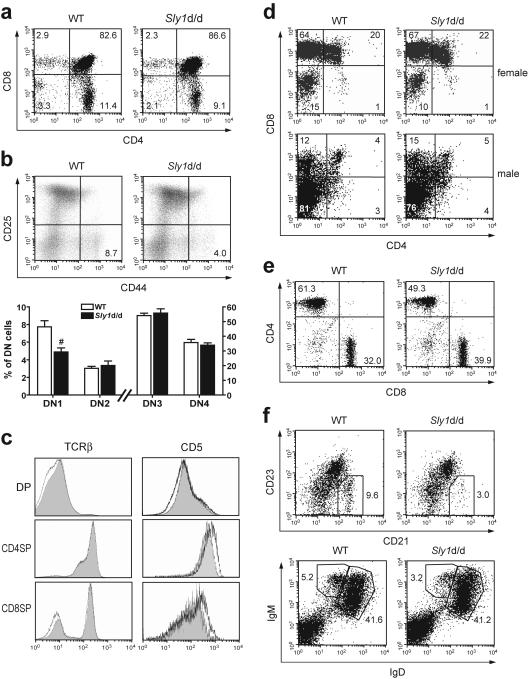
FIG. 3.
Development of T and B cells in Sly1d/d mice. (a) Thymocyte subsets were analyzed by flow cytometry using antibodies against CD4 and CD8. (b) Subsets of double-negative thymocytes were further differentiated by their CD44 and CD25 expression profile (upper panels). In the lower panel, results from eight to nine independent mice per group are summarized. (c) Histograms depict surface levels of T-cell receptor β(TCRβ) and CD5 on double-positive (DP) and single-positive (SP) thymocytes (wild type [WT], shaded graphs; Sly1d/d mice, black lines). (d) Sly1d/d mice were mated with mice expressing a transgenic T-cell receptor specific for the HY antigen in the context with H2-Db. Thymocytes were gated for HY-TCRhigh cells and the proportions of CD4- and CD8-defined subsets present in female and male mice are shown. (e) The proportions of CD4 and CD8 T cells (gated on CD3+ cells) and (f) CD21hi CD23lo (gated on IgM+ cells) or IgMhi IgDlo marginal zone B cells in spleens of wild-type and Sly1d/d mice were determined. All plots are representative of at least 6 independent mice per group. #, P < 0.01 (Student's t test).
The maturation of double-negative (DN) thymocytes progresses from immature CD25− CD44+ (DN1) progenitors to CD25+ CD44+ (DN2) and CD25+ CD44− (DN3) transitional stages, before developing into CD25− CD44− (DN4) thymocytes. The data in Fig. 3b show that although Sly1d/d mice have normal numbers of double-negative thymocytes the proportion of DN1 cells, but not other double-negative subsets, was significantly reduced. During subsequent selection processes double-positive thymocytes differentiate into single-positive cells thereby progressively upregulating expression of the T-cell receptor and of CD5 (3, 22, 33).
We observed comparable expression of the T-cell receptor β-chain and CD5 on double-positive and single-positive thymocyte subsets from Sly1d/d and wild-type mice (Fig. 3c) suggesting that the Sly1d/d mutation does not affect thymic positive selection. To further investigate the role of SLY1 for thymic selection, Sly1d/d mice were crossed with transgenic mice expressing a T-cell receptor specific for the male antigen HY in the context of H-2Db. Thymocytes that express the HY-specific T-cell receptor are negatively selected in male H-2b mice resulting in a strong reduction of double-positive and CD8 single-positive thymocytes, whereas thymocytes from female transgenic mice are positively selected on an H-2b background and develop into mature CD8 single-positive cells (14). The data in Fig. 3d show that the Sly1d/d mutation did not influence the development of double-positive or CD8 single-positive thymocytes expressing the HY-specific T-cell receptor irrespective of whether analyses were performed in female or male mice. These results therefore show that thymic positive and negative selection is not altered in Sly1d/d mice.
Further analysis of peripheral T cells in spleen revealed that both the proportion and the absolute numbers of CD4+ cells were reduced in Sly1d/d compared to wild-type mice (Table 1 and Fig. 3e). In contrast, the proportion of CD8 T cells was increased in Sly1d/d mice and the absolute numbers were comparable in Sly1d/d and wild-type mice. Similar results were obtained upon analysis of T-cell subsets in peripheral lymph nodes (data not shown). Both CD4 and CD8 wild-type and Sly1d/d T cells did not differ in their surface expression of CD3, CD28, CD25, CTLA4, CD62L, and CD69 (data not shown). Moreover, SLY1 mRNA was found to be expressed in both CD4 and CD8 T-cell subsets (data not shown). The subset of CD4 T cells expressing CD25, which is known to include regulatory T cells (18, 28, 30), was not significantly altered by the Sly1d/d mutation (Table 1).
Within the B-cell lineage, the Sly1d/d mutation caused a marked reduction in the fraction of splenic marginal zone B cells, which were defined by their IgMhi IgDlo or IgM+ CD21hi CD23lo phenotype (Table 1 and Fig. 3f). Notably, the absolute numbers of splenic marginal zone B cells were reduced in Sly1d/d mice by about 63% when compared to wild-type controls. In contrast, Sly1d/d mice had normal proportions and numbers of splenic follicular IgMlo IgDhi B cells as well as peritoneal B1 cells (Table 1, Fig. 3f and 2a). Moreover, the proportions of precursor B-cell subsets in bone marrow were not influenced by Sly1 mutation (Table 1). Surface levels of IgM and IgD were not different between wild-type and Sly1d/d B cells (Fig. 3f, lower panel). Thus, the Sly1d/d mutation resulted in a marked hypoplasia of various lymphoid organs and impaired the development and/or maintenance of DN1, double-positive, and CD4 single-positive thymocytes, the peripheral CD4 T-cell subset, and splenic marginal zone B cells.
Effects of Sly1d/d mutation on B-cell activation and humoral immune responses.
The Sly1d/d mutation affected generation of marginal zone, but not follicular, B cells. To examine the influence of the Sly1d/d mutation on B-cell function, B-cell subsets were purified and cell proliferation was measured after cross-linking of the antigen receptor by antibodies to IgM in the presence or absence of CD40 costimulation. The results in Fig. 4a show that antibody-mediated cross-linking of IgM and CD40 induced comparable proliferation of follicular B cells from Sly1d/d and wild-type mice, whereas anti-IgM alone was not sufficient to stimulate proliferation. Wild-type and Sly1d/d follicular B cells also exhibited comparable proliferation upon exposure to lipopolysaccharide, which is known to engage Toll-like receptor 4 (Fig. 4a). In contrast, proliferation of purified Sly1d/d marginal-zone B cells in response to IgM and CD40 ligation was strongly reduced when compared to wild-type cells (Fig. 4b).
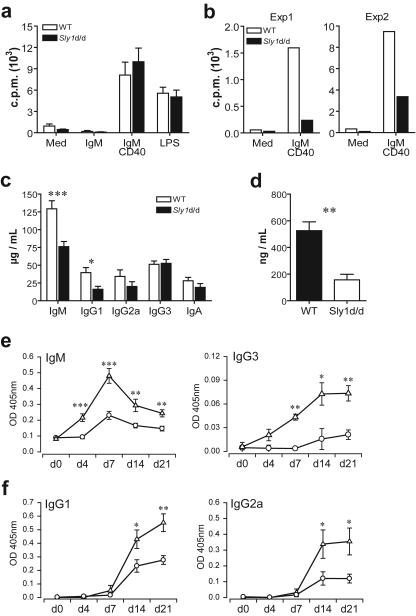
FIG.4.
B-cell activation and humoral immune responses in Sly1d/d mice. B220+ CD21int CD23hi follicular B cells (a) and B220+ CD21hi CD23lo marginal zone B cells (b) were purified from spleens of wild-type (white bars) and Sly1 mutant (solid bars) mice and were stimulated with anti-IgM and anti-CD40 or 40 ng/ml lipopolysaccharide (LPS). [3H]thymidine incorporation was measured after 3 days (n = 4 experiments). (c) Basal levels of immunoglobulin isotypes were determined in serum of untreated mice (n = 17 to 19 mice per group). (d) Levels of IgM in the peritoneal lavage (n = 8 to 9 mice per group). (e) For analysis of T-independent antibody responses, the time course of TNP-specific IgM and IgG3 antibody levels was determined in serum of wild-type (triangles) and Sly1 mutant (circles) mice immunized with TNP-Ficoll (n = 7 to 8 mice per group). (f) T-dependent production of TNP-specific antibody isotypes was measured after immunization of mice with TNP-CGG adsorbed to alum (n = 4 mice per group for day 4 and day 7; n = 8 mice per group for day 0, day 14, and day 21). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test).
Next, we examined whether the Sly1d/d mutation would affect basal immunoglobulin levels in serum of mice. The results in Fig. 4c demonstrate that serum levels of IgM and IgG1 were significantly lower in Sly1d/d mutant mice than in wild-type littermates. In contrast, serum levels of IgG2a, IgG3, and IgA isotypes were not significantly altered by the Sly1d/d mutation. Although peritoneal B1 cell numbers were normal in Sly1d/d mice, reduced serum IgM levels suggested that the antibody-producing capacity of these cells might be impaired, as peritoneal B1 cells constitutively secrete immunoglobulins and are considered a major source of serum IgM (8, 13). To further investigate the influence of the Sly1d/d mutation on B1 cell function, IgM levels were measured in peritoneal lavage fluid. As shown in Fig. 4d, peritoneal IgM levels were markedly reduced in Sly1d/d mice compared to wild-type controls.
Mice expressing mutant SLY1 exhibit a marked reduction of marginal zone B cells, which are known to play an important role in humoral immune responses to T-cell-independent antigens (8, 19). We therefore examined the antibody response ofSly1d/d mice against the T-cell-independent antigen TNP- Ficoll. The results in Fig. 4e demonstrate that production of TNP-specific IgM antibodies was severely reduced in Sly1d/d mutant mice compared to wild-type littermates at all time points studied. At the peak of the T-independent antibody response (day 7), antigen-specific IgM levels were reduced in Sly1d/d mice by greater than 80%. In addition, TNP-Ficoll stimulated production of antigen-specific IgG3 antibodies was markedly attenuated in Sly1d/d compared to wild-type mice (Fig. 4e).
Sly1d/d mutant mice exhibited reduced basal levels of serum IgG1, whose production is dependent on T-cell help to B cells. We therefore investigated whether the Sly1d/d mutation might also influence T-dependent humoral immune responses. Mice were immunized with TNP-CGG (chicken gammaglobulin) adsorbed to alum as an adjuvant and TNP-specific antibodies were measured. Following TNP-CGG immunization very low levels of antigen-specific IgM antibodies were detected in both groups of mice (data not shown). The results in Fig. 4f demonstrate that the levels of IgG1 as well as IgG2a antibodies against TNP were substantially reduced in Sly1d/d mice compared to wild-type controls. Thus, T-cell-dependent humoral immune responses are also markedly attenuated in Sly1d/d mice. Because the Sly1d/d mutation did not affect surface IgM-triggered proliferation of follicular B cells, the data also suggest the possibility that, in addition to reduced cell numbers, CD4 T-cell function might be impaired in Sly1d/d mice.
Impaired antigen receptor-mediated activation of T cells from Sly1d/d mice.
To directly investigate the influence of the Sly1d/d mutation on T-cell responses, we stimulated splenic Tcells in vitro by incubation with antibodies to CD3. The production of IL-2, IL-10, tumor necrosis factor, and the Th1- and Th2-type cytokines gamma interferon and IL-4 by Sly1d/d mutant splenocytes was severely impaired compared to wild-type cells (Fig. 5a). Cytokine production was reduced to a similar extent when Sly1d/d T cells were activated in the presence or absence of anti-CD28 antibodies suggesting that costimulation through CD28 cannot compensate for the defect caused by the Sly1d/d mutation. In contrast to T-cell receptor ligation, stimulation with phorbol myristate acetate and calcium ionophore resulted in a comparable production of IL-2 by wild-type and Sly1d/d T cells (Fig. 5a).
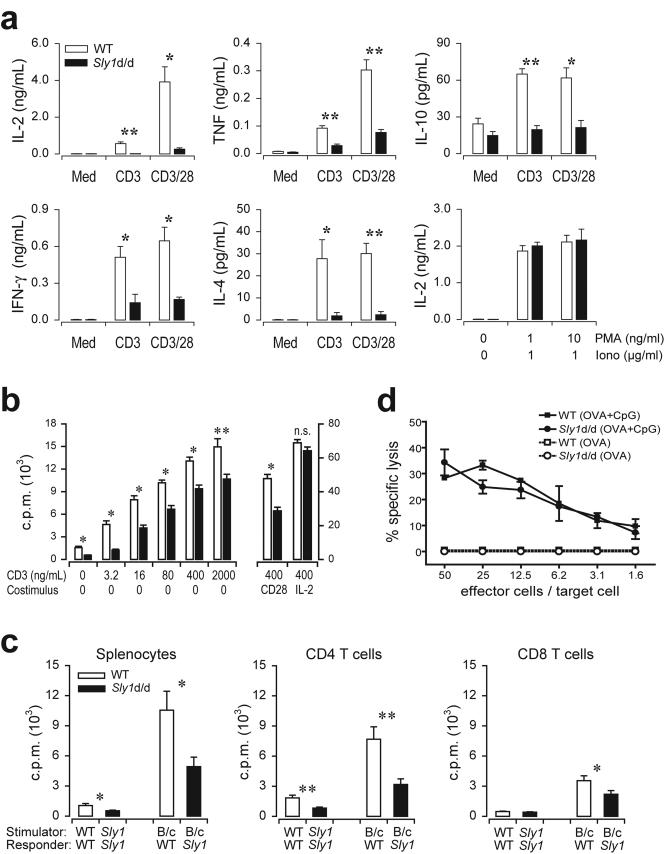
FIG. 5.
Impaired antigen receptor-mediated activation of Sly1d/d T cells. (a) Splenocytes from wild-type (white bars) or Sly1d/d mice (solid bars) were stimulated with CD3 (2 μg/ml) alone or a combination of CD3 (0.4 μg/ml) and CD28 (2.5 μg/ml) antibodies and cytokine production was measured after 18 h. Additionally, IL-2 release was determined upon stimulation with the indicated concentrations of phorbol myristate acetate and ionomycin (n = 4 to 6 mice per group). (b) Splenocytes from wild-type (white bars) or Sly1d/d mice (solid bars) were stimulated by the addition of antibodies against CD3. CD28 antibodies (2.5 μg/ml) or IL-2 (10 ng/ml) were added as indicated. After 66 h [3H]thymidine was added and incorporation was measured after additional 16 h (n = 12 mice per group). (c) Total splenocytes or purified splenic CD4 and CD8 T cells were prepared from Sly1d/d or wild-type (WT) mice and stimulated with allogeneic BALB/c splenocytes (B/c) or autologous cells (n = 5 mice per group). (d) Mice were immunized with ovalbumin in the presence or absence of CpG DNA (CpG) as an adjuvant. Draining lymph node cells were harvested 4 days later and cultured in vitro with recombinant IL-2 for an additional 4 days. Cytotoxic T-cell activity was assayed using EL4 target cells pulsed with the SIINFEKL peptide (n = 4 independent experiments). *, P < 0.05, and **, P < 0.01 (Student's t test), for all experiments.
In additional experiments, the proliferative response of splenic T cells stimulated with anti-CD3 antibodies was examined. The dose-response curve depicted in Fig. 5b demonstrates that, at all concentrations tested, T-cell proliferation was significantly attenuated by the Sly1d/d mutation. CD3-driven proliferation of Sly1d/d T cells was also markedly impaired in the presence of anti-CD28 antibodies. In contrast to CD28 costimulation, the addition of exogenous IL-2 compensated for the effect of the Sly1d/d mutation resulting in normal proliferation (Fig. 5b). Because IL-2 activity requires CD25 expression, cell surface levels of CD25 were measured following CD3 stimulation. We found that upregulation of CD25 was comparable between wild-type and Sly1d/d CD4 and CD8 Tcells (data not shown). Similarly, the Sly1d/d mutation did not influence CD3-stimulated expression of CD69 (data not shown). Together, these results suggest that in vitro T-cell proliferation in response to antigen-receptor ligation is markedly impaired by the Sly1d/d mutation and that this defect may be attributed, at least in part, to reduced IL-2 production.
The role of SLY1 for T-cell responses was further investigated in allogeneic mixed lymphocyte reactions using irradiated BALB/c splenocytes as stimulator cells. As shown in Fig. 5c the proliferation of Sly1d/d T cells was significantly reduced compared to wild-type T cells. To further distinguish the effects of the Sly1d/d mutation on CD4 and CD8 T-cell subsets, purified CD4 and CD8 T cells were used as responder cells. We found that the Sly1d/d mutation severely attenuated the proliferative response of CD4 T cells, whereas CD8 T-cell proliferation was only moderately affected (Fig. 5c). To investigate the function of SLY1 for CD8 T cells in more detail, mice were immunized with ovalbumin and CpG-DNA as adjuvant and cytotoxic T-cell activity was determined in vitro. Under these conditions of immunization, the generation of antigen-specific cytotoxic T cells is independent of CD4 T cells (5, 32). Sly1d/d mice and wild-type controls were able to mount comparable cytotoxic T-cell activity (Fig. 5d). These results therefore indicate that SLY1 is essential for the proliferation and induction of certain effector functions of T cells with more pronounced effects on the CD4 cell subset.
Prolonged allograft survival in Sly1d/d mice.
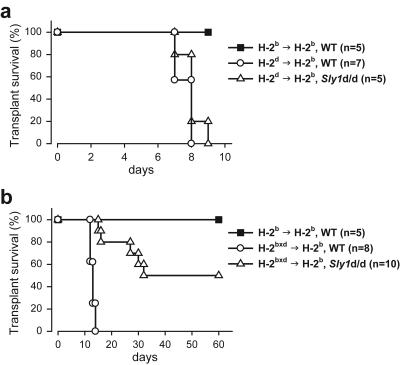
The Sly1d/d mutation caused impaired T-cell cytokine production and attenuated the proliferative response of T cells to allogeneic stimulator cells in vitro. To investigate whether the mutation would also affect T-cell responses in vivo, a model of heterotopic heart transplantation was examined. The results in Fig. 6a demonstrate that Sly1d/d mutant mice (H-2b) rejected fully allogeneic heart transplants (H-2d) as efficiently as wild-type hosts (H-2b). Control syngeneic grafts were not rejected during the observation period.
FIG. 6.
Improved cardiac allograft acceptance in Sly1d/d mice. Fully allogeneic H-2d (a) or semi-identical H-2bxd (b) cardiac allografts were transplanted into wild-type or Sly1 mutant mice. For control, wild-type mice received syngeneic H-2b grafts. Allograft survival was monitored for the indicated time periods.
The activation of alloreactive T cells, which mediate acute transplant rejection, involves multiple pathways that act, at least in part, in a redundant fashion. During rejection of fully allogeneic grafts, help provided by activated NK and/or NK T cells is sufficient to overcome defects of T-cell activation such as those caused by CD28 deficiency (17). Thus, the influence of an individual pathway of T-cell activation on transplant rejection may only become apparent in the absence of help derived from NK-receptor-bearing cells. Because recognition of self major histocompatibility complex class I molecules is known to prevent activation of NK cells, we transplanted cardiac allografts semi-identical in haplotype (H-2bxd) into wild-type and Sly1d/d mutant mice (both H-2b). Consistent with our previous work (17), semi-identical H-2bxd transplants were acutely rejected by H-2b wild-type hosts (Fig. 6b). In marked contrast, transplant survival was significantly prolonged in Sly1d/d mice (Fig. 6b). Half of the semi-identical hearts transplanted into Sly1d/d hosts survived longer than 60 days and half of the grafts were rejected with delayed kinetics compared to wild-type hosts. These results therefore indicate that the Sly1d/d mutation substantially improves acceptance of cardiac allografts in the absence of NK cell help.
Functional T-cell receptor signaling in Sly1d/d lymphocytes.
Considering that SLY1 is phosphorylated upon T-cell receptor triggering (2), we determined whether receptor-proximal signaling events would be altered in Sly1d/d mutant cells. Engagement of the T-cell receptor by antigen or antibody-cross-linking causes immediate increases of intracellular Ca2+ levels. However, the data in Fig. 7a show that stimulation of CD4 and CD8 T cells with CD3 antibodies results in comparable Ca2+ flux of wild-type and Sly1d/d cells. To examine the effects of the Sly1d/d mutation on T-cell receptor-triggered tyrosine-phosphorylation purified splenic T cells were stimulated with CD3 antibodies for the indicated time points and Western blot analyses were performed.
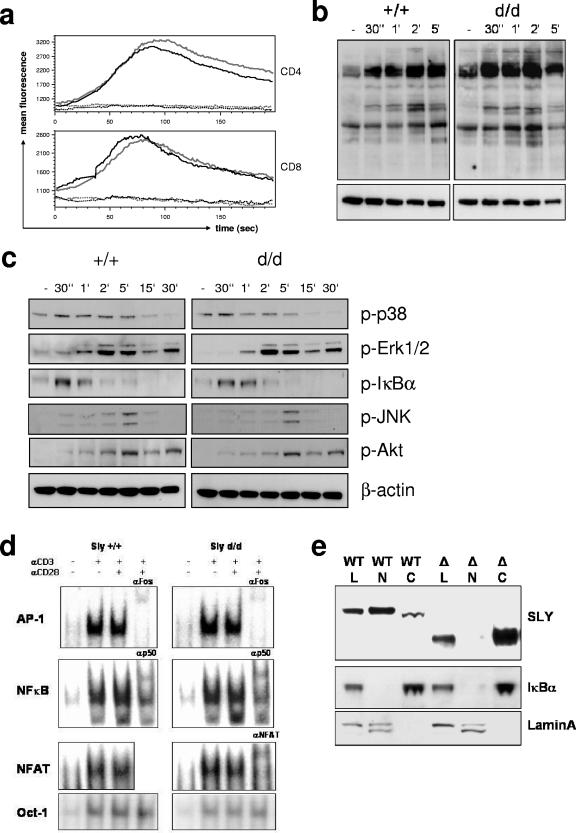
FIG. 7.
Influence of the Sly1d/d mutation on T-cell receptor signaling and subcellular localization of SLY1 protein. (a) Splenocytes were loaded with Fluo-4 and stained for CD4 and CD8 on ice. After warming, baseline fluorescence was determined and T-cell receptor signaling was induced by CD3 cross-linking. (b) T cells were stimulated with anti-CD3 for the indicated periods. Cell lysates were analyzed by Western blotting with antibodies against phosphotyrosine, as well as (c) phospho-p38, phospho-Erk1/2, phospho-JNK, phospho-Akt, and phospho-IκBα as indicated. Blots were stripped and reprobed with anti-β-actin as a loading control. (d) T cells were stimulated for 16 h as indicated and mobility shift assays with specific oligonucleotides were performed. Antibodies used for supershifts are indicated. (e) For subcellular localization of SLY1 protein, whole-cell lysates (L) as well as cytoplasmic (C) and nuclear (N) fractions from lymphocytes were prepared, followed by Western blot analysis with anti-SLY1 (2-16) antibody. The blots were stripped and reprobed with anti-IκBα and anti-lamin A/C antibodies to control for the purity of the preparations.
T-cell receptor-induced tyrosine-phosphorylation in Sly1d/d mutant cells was comparable with phosphorylation in wild-type cells (Fig. 7b). It is well established that upon T-cell receptor triggering membrane-proximal signaling events lead to serine/threonine phosphorylation of several kinases mediating activation and nuclear translocation of transcription factors. However, the Sly1d/d mutation did not appear to influence basal or T-cell receptor-induced phosphorylation of Akt, p38, Erk1/2, JNK, and IκBα (Fig. 7c). In order to investigate whether the translocation of transcription factors would be altered, Sly1d/d mutant cells were stimulated with anti-CD3 and anti-CD28 for 16 h and nuclear fractions were prepared. Consistent with normal mitogen-activated protein kinase activation and IκBα degradation, DNA binding by NF-κB, AP-1, and NF-AT was comparable in wild-type and Sly1d/d T cells (Fig. 7d).
Nuclear localization of SLY1 is abolished by the Sly1d/d mutation.
The Sly1d/d mutation results in the loss of the C-terminal portion of a putative nuclear localization signal (Fig.1a and e). We therefore hypothesized that the Sly1d/d mutation would abrogate nuclear localization thereby impairing SLY1 function. To address this question, cytosolic and nuclear extracts of T lymphocytes were prepared and the presence of SLY1 protein was examined by Western blotting with specific antisera. As shown in Fig. 7e, wild-type SLY1 protein was detected in both the cytoplasm and the nucleus, whereas mutant SLY1d protein was present exclusively in cytoplasmic fractions. The purity of the subcellular fractions was confirmed by Western blotting with antibodies against IκBα (cytosolic fractions) and lamin A/C (nuclear fractions). Thus, the nuclear localization of SLY1 and/or the phosphorylation of Ser27 are essential for its physiological function.
DISCUSSION
The present report extends the current knowledge about the molecular constituents of adaptive immunity by demonstrating an essential function for SLY1, which was identified as a member of a novel family of SH3 and SAM domain-containing proteins. We show that mutation of the SLY1 protein affects the development of CD4 T cells, T-cell proliferation, and cytokine production, the development and proliferation of marginal zone B cells, and the generation of humoral immune responses. Survival of cardiac allografts was prolonged in Sly1d/d mice. While wild-type SLY1 protein was localized in both cytoplasmic and nuclear fractions of T cells, mutant SLY1 was present exclusively in the cytosol. Ablation of nuclear localization of SLY1 by the Sly1d/d mutation is consistent with the deletion of the C-terminal portion of the nuclear localization signal. The observed absence of the mutant SLY1d protein in the nucleus and the phenotype of the Sly1d/d mice suggest that SLY1 could serve as a molecular shuttle in between nuclear and cytoplasmic compartments needed for full activation of lymphocytes.
Although cytokine production upon T-cell receptor triggering in Sly1d/d cells was strongly reduced, membrane-proximal events such as Ca2+ influx, tyrosine phosphorylation, and activation of mitogen-activated protein kinases were not altered. Moreover, translocation of transcription factors such as NF-κB, NFAT, and AP-1 was comparable. It is intriguing to speculate whether the physiological function of SLY1 is to mediate transport of as yet unidentified interaction partners from the cytoplasm to the nucleus or vice versa. Further studies are required to address this question, in particular the nature of the possible interacting molecule and the role of the SH3 and SAM domains in this context. Furthermore, it has to be determined whether the lack of Ser27 phosphorylation or the defective function of the nuclear localization signal or both contribute to the observed functional deficiencies in Sly1d/d mice. Collectively, the results obtained in the Sly1d/d mice indicate that the expression of intact SLY1 protein and its localization to the nucleus are required for the generation of adaptive immune responses.
CD4 T cells are known to be of paramount importance for allograft rejection (27). Activated CD4 T cells direct the immune response against allografts by secreting cytokines that activate and attract effector cells such as CD8 T cells, B cells, and NKT cells (37). Thus, the markedly prolonged survival of semi-identical allografts in Sly1d/d mice may be explained, at least in part, by the fact that the described mutation of SLY1 leads to attenuated proliferation and cytokine production of Tcells upon antigen receptor ligation and an impaired capacity of CD4 T cells to respond to allogeneic stimuli. Activation of allograft-reactive CD4 T cells may occur through multiple pathways that involve an intense interplay of innate and adaptive immune reactions. Thus, NK receptor-bearing cells become activated upon transplantation of fully allogeneic grafts and provide help to T cells, which subsequently mediate acute transplant rejection (17). Importantly, help provided by activated NK and/or NKT cells may be sufficient to overcome defects of T-cell costimulation such as those caused by CD28 deficiency (17).
These results clearly established that the pathways for the induction of T-cell responses to allografts are, at least in part, functionally redundant. Therefore, the influence of individual regulators of T-cell activation such as SLY1 on transplant rejection may only become apparent after reducing this complexity. To avoid help derived from NK-receptor-bearing cells, we transplanted semi-identical (H-2bxd) cardiac allografts into wild-type (H-2b) and Sly1d/d mice (H-2b). We show that the acceptance of semi-identical grafts was substantially extended in Sly1d/d hosts with 50% of the grafts surviving even after 60days. Fully allogeneic grafts, however, were vigorously rejected in Sly1d/d mice. The phenotype of Sly1d/d mice in transplantation is therefore similar to that of CD28-null mice, which also accept semi-identical cardiac transplants but efficiently reject fully allogeneic grafts (17).
In Sly1d/d mice, the antibody response to T-dependent as well as T-independent antigens was severely impaired thereby identifying SLY1 as a novel molecular regulator of humoral immune responses. T-dependent antibody responses may be affected by the Sly1d/d mutation at multiple levels. Efficient B-cell activation by T-dependent antigens requires signals delivered through binding of the antigen to the B-cell receptor as well as generation of antigen-specific T helper cells that provide costimulatory signals to B cells via CD40-CD40 ligand interactions and the secretion of cytokines. Following antigen receptor ligation, the proliferative response of CD4 T cells derived from Sly1d/d mice was markedly diminished, whereas proliferation of follicular B2 B cells was not affected. In addition, Sly1d/d T cells exhibited reduced secretion of cytokines that are required as growth and differentiation factors for proliferation and isotype switching of antigen-reactive B cells. Consistent with a diminished production of IL-4 and gamma interferon, both IgG1 and IgG2a responses to T-dependent antigen were diminished in Sly1d/d mice. It is therefore conceivable that T-dependent antibody responses may be impaired in Sly1d/d mice due to the lack of sufficient T-cell help.
T-independent antibody responses are considered important for host defenses as they bridge early innate to subsequently appearing adaptive immune reactions (8). In addition to B1 cells, marginal-zone B cells play a major role for the first-line responses against blood-borne T-independent antigens. Evidence for this notion is provided by analysis of Pyk-2-deficient mice, which exhibit a selective defect in marginal zone B-cell development that is associated with severely impaired T-independent antibody responses (11). Subsequent studies with B-cell receptor transgenic mice directly demonstrated that marginal zone B cells efficiently generate antigen-specific plasma blasts after immunization with T-independent antigens (20). It therefore appears likely that the impaired T-independent antibody response in Sly1d/d mice may result, at least in part, from severely reduced numbers and a substantial reduction of antigen receptor-driven proliferation of Sly1d/d marginal zone Bcells.
After emigration of newly formed surface IgM+ B cells from the bone marrow to peripheral lymphoid organs the antigen receptor signal strength determines cell fate decision to follicular or marginal zone B cells (23). Genetic ablation of CD19, Lyn, or the p110δ subunit of phosphatidylinositol 3-kinase, all of which influence B-cell receptor signaling, impairs generation and/or positive selection of marginal zone B cells, whereas PTEN deficiency, which results in enhanced B-cell receptor signaling, is characterized by increased numbers of marginal zone B cells. It has been shown that other genes unrelated to B-cell receptor signaling such as Pyk-2, Lsc, or NF-κB p50 are also indispensable for marginal-zone B-cell development, suggesting that, in addition to quantitative aspects of B-cell receptor signaling, qualitatively distinct pathways of B-cell activation may exist (10, 23). It is unclear, if the preferential reduction of marginal zone versus follicular B-cell numbers in Sly1d/d mice is due to modified B-cell receptor signaling, because downstream signals are not altered (data not shown).
Few genes on the X chromosome encompassing Btk, SH2D1A, and CD40 ligand are linked to genetic immune disorders. Mutations in Btk result in X-linked agammaglobulinemia (34, 36), X-linked lymphoproliferative disease is a result of mutations in the SH2D1A gene, whereas CD40 ligand deficiency causes X-linked hyper-IgM syndrome (7, 26, 38). In addition, there are several X-linked immune diseases with unidentified corresponding genetic defects. The results of the present study demonstrate that mutations in the Sly1 gene can lead to multiple defects in B- and T-cell responses consistent with a common variable immunodeficiency syndrome. Gene mutations affecting the function of SLY1 may therefore represent a novel type of X-linked immunodeficiency.
Acknowledgments
This work was supported by grants HO 1015/5-3 (to B.H.) and PF 259/2-5, SFB 575, 576, and 590 (to K.P.) of the Deutsche Forschungsgemeinschaft (DFG), the Kommission für Klinische Forschung, Klinikum rechts der Isar (to B.H.), and the Forschungskommission of Heinrich Heine University (to S.B.).
We thank Bernadett Bartsch, Evelyn Schaller, Anja Conrad, Jennifer Meinecke, and Karin Buchholz for excellent technical assistance, Irmgard Förster for critical reading of the manuscript, Markus Moser for support with the generation of SLY1-specific antisera, and Tatjana Dorn and Cord Brakebusch for help with the Ca2+ flux assays.
We declare that we have no financial or other conflicts of interest.
REFERENCES
- 1.Alimzhanov, M. B., D. V. Kuprash, M. H. Kosco-Vilbois, A. Luz, R. L. Turetskaya, A. Tarakhovsky, K. Rajewsky, S. A. Nedospasov, and K. Pfeffer. 1997. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc. Natl. Acad. Sci. USA 94:9302-9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astoul, E., A. D. Laurence, N. Totty, S. Beer, D. R. Alexander, and D. A. Cantrell. 2003. Approaches to define antigen receptor induced serine kinase signal transduction pathways. J. Biol. Chem. 278:9267-9275. [DOI] [PubMed] [Google Scholar]
- 3.Azzam, H. S., A. Grinberg, K. Lui, H. Shen, E. W. Shores, and P. E. Love. 1998. CD5 expression is developmentally regulated by T-cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188:2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer, S., A. B. Simins, A. Schuster, and B. Holzmann. 2001. Molecular cloning and characterization of a novel SH3 protein (SLY) preferentially expressed in lymphoid cells. Biochim. Biophys. Acta 1520:89-93. [DOI] [PubMed] [Google Scholar]
- 5.Cho, H. J., K. Takabayashi, P. M. Cheng, M. D. Nguyen, M. Corr, S. Tuck, and E. Raz. 2000. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat. Biotechnol. 18:509-514. [DOI] [PubMed] [Google Scholar]
- 6.Claudio, J. O., Y. X. Zhu, S. J. Benn, A. H. Shukla, C. J. McGlade, N. Falcioni, and A. K. Stewart. 2001. HACS1 encodes a novel SH3-SAM adaptor protein differentially expressed in normal and malignant hematopoietic cells. Oncogene 20:5373-5377. [DOI] [PubMed] [Google Scholar]
- 7.Coffey, A. J., R. A. Brooksbank, O. Brandau, T. Oohashi, G. R. Howell, J. M. Bye, A. P. Cahn, J. Durham, P. Heath, P. Wray, R. Pavitt, J. Wilkinson, M. Leversha, E. Huckle, C. J. Shaw-Smith, A. Dunham, S. Rhodes, V. Schuster, G. Porta, L. Yin, P. Serafini, B. Sylla, M. Zollo, B. Franco, A. Bolino, M. Seri, A. Lanyi, J. R. Davis, D. Webster, A. Harris, G. Lenoir, G. de St Basile, A. Jones, B. H. Behloradsky, H. Achatz, J. Murken, R. Fassler, J. Sumegi, G. Romeo, M. Vaudin, M. T. Ross, A. Meindl, and D. R. Bentley. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129-135. [DOI] [PubMed] [Google Scholar]
- 8.Fagarasan, S., and T. Honjo. 2000. T-independent immune response: New aspects of B-cell biology. Science 290:89-92. [DOI] [PubMed] [Google Scholar]
- 9.Garside, P., E. Ingulli, R. R. Merica, J. G. Johnson, R. J. Noelle, and M. K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281:96-99. [DOI] [PubMed] [Google Scholar]
- 10.Girkontaite, I., K. Missy, V. Sakk, A. Harenberg, K. Tedford, T. Pötzel, K. Pfeffer, and K.-D. Fischer. 2001. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2:855-862. [DOI] [PubMed] [Google Scholar]
- 11.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B cells in pyk-2-deficient mice defines their role in humoral immune responses. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, M. S., A. L. Singer, and G. A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110-116. [DOI] [PubMed] [Google Scholar]
- 13.Kantor, A. B., and L. A. Herzenberg. 1993. Origin of murine B-cell lineages. Annu. Rev. Immunol. 11:501-538. [DOI] [PubMed] [Google Scholar]
- 14.Kisielow, P., H. Bluthmann, U. D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333:742-746. [DOI] [PubMed] [Google Scholar]
- 15.Koni, P. A., R. Sacca, P. Lawton, J. L. Browning, N. H. Ruddle, and R. A. Flavell. 1997. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity 6:491-500. [DOI] [PubMed] [Google Scholar]
- 16.Kurosaki, T. 2002. Regulation of B-cell signal transduction by adaptor proteins. Nat. Rev. Immunol. 2:354-363. [DOI] [PubMed] [Google Scholar]
- 17.Maier, S., C. Tertilt, N. Chambron, K. Gerauer, N. Hüser, C. D. Heidecke, and K. Pfeffer. 2001. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat. Med. 7:557-562. [DOI] [PubMed] [Google Scholar]
- 18.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 19.Martin, F., and J. F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323-335. [DOI] [PubMed] [Google Scholar]
- 20.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, K. M., and S. L. Reiner. 2002. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2:933-944. [DOI] [PubMed] [Google Scholar]
- 22.Ohashi, P. S., H. Pircher, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1990. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature 346:861-863. [DOI] [PubMed] [Google Scholar]
- 23.Pillai, S., A. Cariappa, and S. T. Moran. 2005. Marginal zone B cells. Annu. Rev. Immunol. 23:161-196. [DOI] [PubMed] [Google Scholar]
- 24.Re, D., P. Starostik, N. Massoudi, A. Staratschek-Jox, V. Dries, R. K. Thomas, V. Diehl, and J. Wolf. 2003. Allelic losses on chromosome 6q25 in hodgkin and reed sternberg cells. Cancer Res. 63:2606-2609. [PubMed] [Google Scholar]
- 25.Rennert, P. D., J. L. Browning, R. Mebius, F. Mackay, and P. S. Hochman. 1996. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 184:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renshaw, B. R., W. C. Fanslow, R. J. Armitage, K. A. Campbell, D. Liggitt, B. Wright, B. L. Davison, and C. R. Maliszewski. 1994. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 180:1889-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg, A. S., and A. Singer. 1992. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu. Rev. Immunol. 10:333-358. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531-562. [DOI] [PubMed] [Google Scholar]
- 29.Samelson, L. E. 2002. Signal transduction mediated by the T-cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 20:371-394. [DOI] [PubMed] [Google Scholar]
- 30.Shevach, E. M. 2002. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389-400. [DOI] [PubMed] [Google Scholar]
- 31.Simeoni, L., S. Kliche, J. Lindquist, and B. Schraven. 2004. Adaptors and linkers in T and B cells. Curr. Opin. Immunol. 16:304-313. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser, T., R. M. Vabulas, B. Villmow, G. B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T-cell responses to soluble proteins. Eur. J. Immunol. 30:3591-3597. [DOI] [PubMed] [Google Scholar]
- 33.Swat, D., M. Dessing, A. Baron, P. Kisielow, and H. von Boehmer. 1992. Phenotypic changes accompanying selection of CD4+CD8+ thymocytes. Eur. J. Immunol. 22:2367-2372. [DOI] [PubMed] [Google Scholar]
- 34.Tsukada, S., D. C. Saffran, D. J. Rawlings, O. Parolini, R. C. Allen, I. Klisa, R. S. Sparkes, H. Kubagawa, T. Mohandas, S. Quan, and et al. 1993. Deficient expression of a B-cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72:279-290. [DOI] [PubMed] [Google Scholar]
- 35.Uchida, T., A. Nakao, N. Nakano, A. Kuramasu, H. Saito, K. Okumura, and H. Ogawa. 2001. Identification of Nash1, a novel protein containing a nuclear localization signal, a sterile α motif, and an SH3 domain preferentially expressed in mast cells. Biochem. Biophys. Res. Commun. 288:137-141. [DOI] [PubMed] [Google Scholar]
- 36.Vetrie, D., I. Vorechovsky, P. Sideras, J. Holland, A. Davies, F. Flinter, L. Hammarstrom, C. Kinnon, R. Levinsky, M. Bobrow, and et al. 1993. The gene involved in X-linked agammaglobulinemia is a member of the src family of protein-tyrosine kinases. Nature 361:226-233. [DOI] [PubMed] [Google Scholar]
- 37.Walsh, P. T., T. B. Strom, and L. A. Turka. 2004. Routes to transplant tolerance versus rejection: the roles of cytokine. Immunity 20:121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, J., T. M. Foy, J. D. Laman, E. A. Elliott, J. J. Dunn, T. J. Waldschmidt, J. Elsemore, R. J. Noelle, and R. A. Flavell. 1998. Mice deficient for the CD40 ligand. Immunity 1:423-431. [DOI] [PubMed] [Google Scholar]
- 39.Zeller, C., B. Hinzmann, S. Seitz, H. Prokoph, E. Burkhard-Goettges, J. Fischer, B. Jandrig, L.-E. Schwarz, A. Rosenthal, and S. Scherneck. 2003. SASH1: a candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 22:2972-2983. [DOI] [PubMed] [Google Scholar]