Abstract
Mice with muscle-specific knockout of the Glut4 glucose transporter (muscle-G4KO) are insulin resistant and mildly diabetic. Here we show that despite markedly reduced glucose transport in muscle, muscle glycogen content in the fasted state is increased. We sought to determine the mechanism(s). Basal glycogen synthase activity is increased by 34% and glycogen phosphorylase activity is decreased by 17% (P < 0.05) in muscle of muscle-G4KO mice. Contraction-induced glycogen breakdown is normal. The increased glycogen synthase activity occurs in spite of decreased signaling through the insulin receptor substrate 1 (IRS-1)-phosphoinositide (PI) 3-kinase-Akt pathway and increased glycogen synthase kinase 3β (GSK3β) activity in the basal state. Hexokinase II is increased, leading to an approximately twofold increase in glucose-6-phosphate levels. In addition, the levels of two scaffolding proteins that are glycogen-targeting subunits of protein phosphatase 1 (PP1), the muscle-specific regulatory subunit (RGL) and the protein targeting to glycogen (PTG), are strikingly increased by 3.2- to 4.2-fold in muscle of muscle-G4KO mice compared to wild-type mice. The catalytic activity of PP1, which dephosphorylates and activates glycogen synthase, is also increased. This dominates over the GSK3 effects, since glycogen synthase phosphorylation on the GSK3-regulated site is decreased. Thus, the markedly reduced glucose transport in muscle results in increased glycogen synthase activity due to increased hexokinase II, glucose-6-phosphate, and RGL and PTG levels and enhanced PP1 activity. This, combined with decreased glycogen phosphorylase activity, results in increased glycogen content in muscle in the fasted state when glucose transport is reduced.
A fundamental action of insulin is to control the plasma glucose concentration by stimulating glucose uptake into muscle and adipose cells and inhibiting hepatic glucose output (13). The stimulatory effect of insulin on glucose transport and glycogen synthesis involves a series of cellular events (26, 49). Dysregulation of any of these steps could result in insulin resistance, which is a major risk factor in the development of type 2 diabetes (26, 49). The binding of insulin to its receptor generates intracellular signals that stimulate Glut4 translocation. The nature of the signaling intermediates that mediate this process is under intense investigation (57). It is likely that defects in signaling account for resistance to insulin-stimulated glucose transport in muscle in cases of type 2 diabetes (25, 42, 48).
The relative importance of glucose transport compared to that of glycogen synthase activity in regulating the rate of glycogen synthesis has been debated over many years (32, 44). Attention has focused on the importance of glucose transporters in determining the rate of glycogen synthesis (22, 46). Studies with transgenic mice overexpressing the glucose transporters Glut1 or Glut4 in skeletal muscle support the notion that increasing glucose transport is sufficient to increase glycogen synthesis in spite of the normal activation of glycogen synthase (22, 46). Other evidence, however, suggests that increased activation of glycogen synthase, rather than increased glucose transport, is important for controlling glycogen synthesis in skeletal muscle in vivo (4, 37). The disruption of glycogen synthase in skeletal muscle results in a lack of glycogen accumulation in spite of an increased ability to dispose of glucose (40).
Glycogen content is regulated by the balance between the activities of glycogen synthase and glycogen phosphorylase (52). Glycogen synthase catalyzes the rate-limiting step for glycogen synthesis, while glycogen phosphorylase regulates glycogen breakdown in skeletal muscle (32, 44). Insulin causes the dephosphorylation of both glycogen synthase and phosphorylase in skeletal muscle, resulting in the activation of glycogen synthase and the inactivation of glycogen phosphorylase, thereby increasing glycogen accumulation (32, 39). Glycogen synthase activity is also regulated allosterically by the level of glucose-6-phosphate, in addition to being regulated by the insulin-signaling cascade and by other phosphate compounds (e.g., ATP, ADP, AMP, phosphocreatine) (50). In muscle, glucose-6-phosphate is generated primarily by the action of hexokinase II to phosphorylate glucose. While data show mixed results, depending on the conditions used, a number of studies demonstrate that glucose phosphorylation becomes an important mechanism for the control of muscle glucose uptake during hyperinsulinemia in rodents (18, 43, 64) and humans (6, 7, 27, 58, 59). Furthermore, insulin-induced hexokinase expression is reduced in humans with obesity and type 2 diabetes (41).
Both basal and insulin-stimulated glycogen synthase activation are impaired in muscle of insulin-resistant humans and rodents (24, 29, 56), which might be expected to result in accumulation of less glycogen in muscle. However, high glycogen levels in skeletal muscle are seen in certain insulin-resistant and diabetic animals, such as obese Zucker rats (51), diabetic Zucker rats (51), and rats with streptozotocin-induced diabetes (19). High muscle glycogen levels may actually contribute to insulin resistance, since studies show that increased glycogen content resulting from high-glucose feeding or from muscle denervation impairs insulin signaling in skeletal muscle (15, 34). In addition, high glycogen content inhibits AMP-activated protein kinase activity, which could also contribute to insulin resistance (61). Relatively little is known about the underlying mechanism for increased glycogen content in skeletal muscle in some insulin-resistant states in vivo. Interestingly, in mice lacking Glut4 in all tissues (53), glycogen content levels and glycogen synthesis rates differed between males and females and between different muscle fiber types, being increased in some and decreased in others.
Protein phosphatase 1 (PP1) plays a key role in the regulation of glycogen metabolism, catalyzing the dephosphorylation of glycogen synthase, glycogen phosphorylase, and phosphorylase kinase (9). These dephosphorylation reactions promote the net synthesis of glycogen by activating glycogen synthase and inhibiting phosphorylase (9). The PP1 catalytic subunit (PP1C) interacts with a wide variety of targeting subunits that localize it to specific sites within cells. The muscle-specific regulatory subunit (RGL; also called GM) and protein targeting to glycogen (PTG) are members of a family of glycogen-targeting subunits of PP1 (38, 55). RGL is expressed primarily in skeletal muscle, and PTG is expressed in all insulin-sensitive tissues (38). The binding of RGL or PTG to PP1C enhances the dephosphorylation of glycogen synthase and causes the activation of glycogen synthesis (16, 17, 38). Studies suggest that RGL and PTG could play a crucial role in the regulation of glycogen synthesis. The overexpression of RGL or PTG increases basal glycogen synthase activity and glycogen content in a variety of cell types, such as cultured skeletal muscle cells (33, 45), hepatocytes (5), and CHO cells (35, 62), as well as in skeletal muscle (3) or liver (5, 20) in vivo. In addition, in skeletal muscle of RGL-knockout mice and PTG heterozygous mice, basal glycogen synthase activities and glycogen amounts are reduced (12, 14, 54). However, the full impact of the glycogen-targeting subunits on glycogen metabolism in skeletal muscle in vivo is still unclear.
We previously demonstrated that mice with muscle-specific knockout of Glut4 (muscle-G4KO) are insulin resistant and glucose intolerant from an early age (65). In this study, we investigated the effects of muscle-specific deletion of Glut4 on muscle glycogen metabolism. Here we show that the deletion of Glut4 in muscle results in increased glycogen content in the fasted state in spite of a 75% reduction in glucose transport. Importantly, we find that glycogen synthase activity in muscle is increased in muscle-G4KO mice. This appears to result from the alteration of multiple regulatory steps, including increased hexokinase II leading to increased glucose-6-phosphate levels, increased RGL and PTG resulting in increased PP1 catalytic activity, and decreased glycogen phosphorylase activity. These results indicate that glucose phosphorylation and glycogen-targeting subunits in muscle can be regulated either directly or indirectly when glucose transport is impaired. Furthermore, these steps may play a dominant role in regulating glycogen metabolism in muscle in the fasted state.
MATERIALS AND METHODS
Animals.
All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male or female wild-type (WT) and muscle-specific Glut4-knockout littermates (65) were studied at ∼4 to 12 months of age. The mice were fed standard chow (PMI Feeds, Inc, St. Louis, MO) and housed under controlled-temperature conditions at 24°C and a 12-h light-dark cycle with light from 0630 to 1830 h.
Protocols. (i) Basal state.
After overnight fasting, mice were anesthetized by injection of ketamine and xylazine solution. Skeletal muscles and liver and fat tissue were rapidly removed, frozen in liquid nitrogen, and stored at −80°C until analysis.
(ii) Acute insulin stimulation.
For injection experiments, mice were fasted overnight. On the day of the experiment, a bolus injection of insulin (10 U/kg) was administered through the tail vein. Three min later, skeletal muscles and liver were rapidly removed, frozen in liquid nitrogen, and stored at −80°C until analysis.
(iii) Contraction in isolated muscle.
After a 10- to 12-h fast, mice were killed by cervical dislocation, and soleus muscle was rapidly dissected, tied with suture (silk, size 4-0), and mounted on an incubation apparatus with resting tension set to 0.25 g as described previously (23). Muscles were preincubated in Krebs-Ringer bicarbonate buffer containing 2 mM pyruvate at 37°C for 40 min. When contracted, muscles were electrically stimulated during the last 10 min of this period (train frequency, 2/minute; train duration, 10 seconds; pulse rate, 100/second; pulse duration, 0.1 millisecond; voltage, 100 V). Glycogen content was determined as described below.
Glycogen synthesis in skeletal muscle in vivo.
Mice were injected intraperitoneally with 10 μCi of [U-14C]glucose (ICN Pharmaceuticals Inc.) and sacrificed 1 h later. Samples of plasma were obtained serially at 5, 10, 30, and 60 min after injection for the measurement of plasma glucose levels and specific activities. The muscle was rapidly removed and frozen in liquid N2, and glycogen was extracted as described below. To determine the level of glucose incorporation into muscle glycogen, 14C radioactivity in glycogen (dpm) was divided by the integrated glucose-specific activity area under the curve. Muscle glycogen synthesis was expressed as ng of glucose incorporated into glycogen per mg of muscle per hour.
Preparation of tissue lysates.
Fifty mg of tissue was homogenized using a Polytron device at half the maximum speed for 1 min on ice in 500 μl of buffer A (20 mM Tris, pH 7.5, 5 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4) containing 1% NP-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml aprotinin, and 10 μg/ml leupeptin. For assays of glycogen synthase kinase 3 (GSK3) activity, we changed the concentration of NaF in buffer A from 100 mM NaF to 1 mM NaF. Tissue lysates were solubilized by continuous stirring for 1 h at 4°C and centrifuged for 10 min at 14,000 g. The supernatants were stored at −80°C until analysis.
Determination of glycogen content.
The glycogen content of muscle was determined by modifications of a procedure described by Chan and Exton (10). Muscle was weighed and solubilized with 0.5 N KOH at 95°C. The glycogen was precipitated with 3 volumes of ethanol and 0.1 volume of Na2SO4 (6%) at −80°C. The precipitate was washed with 70% ethanol. The glycogen in the precipitate was digested with amylo-α-1,4-α-1,6-glucosidase (EC 3.2.1.3) in acetate buffer (pH 4.9), and the amount of released glucose was determined by use of a glucose oxidase method (Sigma). Glycogen from rabbit liver (Sigma) was used as the standard.
Determination of glycogen synthase activity.
Twenty milligrams of muscle was homogenized using a Polytron device at half the maximum speed for 1 min on ice in 0.5 ml of extraction buffer (50 mM HEPES, 10 mM EDTA, 100 mM NaF, 5 mM dithiothreitol, 1 μM leupeptin, 1 μM pepstatin, and 200 μM PMSF, pH 7.5). Homogenates were used to measure glycogen synthase activity as described previously (60). Glycogen synthase activity was determined at a physiologic concentration of substrate (0.3 mM UDP-glucose), which was calculated as nanomoles of UDP-glucose incorporated into glycogen per minute per milligram of total protein and expressed as the ratio of activity assayed at 0 mM glucose-6-phosphate divided by the activity at 7.2 mM glucose-6-phosphate. This is an indicator of the change in the phosphorylation state of glycogen synthase in response to insulin (47).
Determination of glycogen phosphorylase activity.
Glycogen phosphorylase activity was measured at 30°C as described previously (21). Phosphorylase measured in the presence of 3 mM AMP is called total activity, whereas activity measured in the absence of added AMP is defined as phosphorylase a activity.
Determination of glucose-6-phosphate concentration.
Glucose-6-phosphate concentration was measured in perchloric acid extracts of frozen muscle according to the method of Lowry and Passonneau (36).
Determination of PP1 activity.
Twenty mg of muscle was homogenized using a Polytron device at half the maximum speed for 1 min on ice in 0.5 ml of PP1 homogenization buffer (50 mM Tris, 2 mM EDTA, 0.2% β-mercaptoethanol, 2 mg/ml glycogen, pH 7.4) containing 0.1 mM PMSF, 1 mM benzamidine, and 10 mg/ml aprotinin. Muscle homogenates (10 μg) were preincubated with PP1 homogenization buffer containing 4.5 nM okadaic acid for 2 min at 37°C. The reaction was initiated by adding 15 μg of 32P-labeled phosphorylase a in the presence of 3 nM okadaic acid and 5 mM caffeine. The level of phosphate release was determined as described previously (8).
Determination of PI3K, Akt, and GSK3 activities.
Muscle lysates (500 μg of protein) were subjected to immunoprecipitation for 4 h at 4°C with either 5 μl of a polyclonal insulin receptor substrate 1 (IRS-1) antibody (1:100 dilution; gift from Morris White, Joslin Diabetes Center), 3 μg of Akt antibody that recognizes both Akt1 and Akt2 (Upstate Biotechnology, Lake Placid, NY), or 5 μl of a polyclonal GSK3β-specific antibody (1:100 dilution; a gift from Hagit Eldar-Finkelman, Tel-Aviv University, Israel) coupled to protein A-Sepharose (Sigma, St. Louis, MO) or protein G-Sepharose beads. The immune complex was washed, and phosphatidylinositol 3-kinase (PI3K), Akt, and GSK3 activities were determined as described previously (30).
Determination of amounts of signaling proteins.
Tissue lysate protein (5 to 50 μg per lane) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 6%, 8%, and 10% gels) and transferred to nitrocellulose membranes (Schleicher & Schuell). The membranes were blocked with 5% nonfat dry milk for 1 h at room temperature and incubated with the following antibodies in 1% nonfat dry milk overnight at 4°C: a polyclonal Glut4 antibody (gift from H. Haspel, Henry Ford Hospital, Detroit, MI), a polyclonal insulin receptor antibody (Santa Cruz Biotechnology, Santa Cruz, CA), a polyclonal IRS-1 antibody (gift from M. White, Joslin Diabetes Center), a polyclonal antibody against the p85α subunit or p110α subunit of PI3K (Upstate Biotechnology), a polyclonal antibody for Akt that recognizes both Akt1 and Akt2 (Upstate Biotechnology), a monoclonal antibody for GSK3 that recognizes both GSK3 α and GSK3 β (Upstate Biotechnology), a polyclonal glycogen synthase antibody (gift from J. Lawerence, University of Virginia), a polyclonal mitogen-activated protein kinase (MAPK) antibody (gift from J. Blenis, Harvard Medical School), a polyclonal PP1 antibody (Upstate Biotechnology), a polyclonal RGL/GM antibody (gift from A. DePaoli-Roach, Indiana University) a polyclonal PTG antibody (gift from M. Brady, University of Chicago), a polyclonal phosphoglycogen synthase (Ser641) antibody (Cell Signaling, Beverly, MA), or a polyclonal hexokinase II (Chemicon, Temecula, CA). The bands were visualized with the enhanced chemiluminescence system (Amersham) and quantified with a densitometer (Molecular Dynamics) (30).
Statistical analysis.
Data are presented as means ± standard errors of the mean (SEM). Statistical analyses were performed using the StatView program (Abacus Concepts, Inc., Berkeley, CA). Statistical significance was tested with the unpaired Student t test or analysis of variance.
RESULTS
Glycogen content.
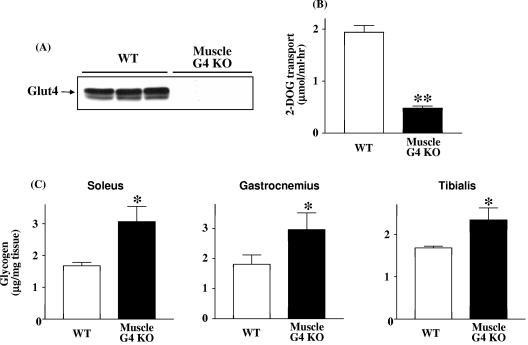
Glut4 protein levels were reduced at least 95% in muscle-G4KO mice (Fig. 1A). Basal glucose transport in vitro was reduced by 75% in soleus muscles of muscle-G4KO mice (Fig. 1B). In spite of markedly reduced glucose transport, glycogen content in the fasted state was increased 83%, 61%, and 39% in soleus, gastrocnemius, and tibialis anterior, respectively, of 4-month-old muscle-G4KO compared with WT mice (P < 0.05) (Fig. 1C). This elevation of glycogen content persisted; in gastrocnemius muscle, glycogen was also elevated by 55% in 8-month-old and by 31% in 12-month-old muscle-G4KO mice compared to WT mice (P < 0.05) (data not shown). Glycogen content in the fed state was unaltered in muscle of muscle-G4KO mice (3.27 ± 0.16 μg/mg) compared with that of WT mice (3.11 ± 0.15 μg/mg) (n = 5; not significant).
FIG. 1.
Glut4 protein (A), basal glucose transport (B), and glycogen content (C) levels in muscle from WT and muscle-G4KO mice. Mice were fasted overnight. (A) Proteins in muscle lysates (25 μg) were separated by SDS-PAGE on 10% gels and transferred to nitrocellulose membranes. Glut4 was visualized by immunoblotting with a Glut4 antibody. (B) Isolated soleus muscles are incubated with 2-deoxy-d-glucose for 20 min. Basal glucose uptake was determined. Results are means ± SEM for three to six mice per group. **, P < 0.01 versus WT. (C) Soleus, gastrocnemius, and tibialis anterior muscle specimens were solubilized with 0.5 N KOH. The glycogen content levels in muscle of WT and muscle-G4KO mice at 4 months of age were determined by use of a glucose oxidase method. Results are means ± SEM for five to six mice per group. *, P < 0.05 versus WT.
The rates of incorporation of [14C]glucose into muscle glycogen were similar in both groups (139 ± 56 ng/mg/hour in WT versus 142 ± 39 ng/mg/hour in muscle-G4KO; n = 6 to 7; not significant), in spite of the fact that basal glucose transport in muscle was 75% lower in muscle-G4KO mice than in WT mice (Fig. 1B) (65). These data suggest that even though only a small amount of glucose is transported into muscle, this glucose is preferentially metabolized to glycogen, resulting in an increase in glycogen synthesis relative to the amount of glucose transported.
Glycogen synthase and glycogen phosphorylase activity.
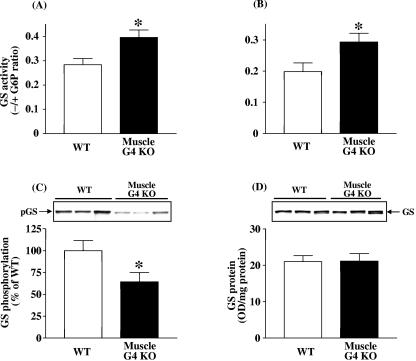
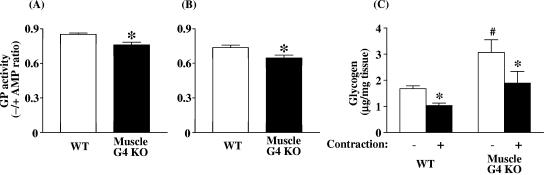
To clarify the mechanism(s) for increased glycogen content, we examined glycogen synthase and glycogen phosphorylase activity in tibialis anterior and gastrocnemius muscle of muscle-G4KO mice. Basal glycogen synthase activity was increased by 40% in tibialis anterior muscle of muscle-G4KO mice and by 47% in gastrocnemius muscle of muscle-G4KO compared with WT mice (P < 0.05) (Fig. 2A and B). Consistent with these findings, the phosphorylation of glycogen synthase on serine 641, which is phosphorylated by GSK3 and critical for glycogen synthase activity, was decreased by 36% in gastrocnemius muscle of muscle-G4KO compared with WT mice (P < 0.05) (Fig. 2C). Figure 2D shows that the protein levels of glycogen synthase in skeletal muscle did not differ between WT and muscle-G4KO mice. Basal glycogen phosphorylase activity levels were slightly decreased by l2% in tibialis anterior muscle and by 14% in gastrocnemius muscle of muscle-G4KO compared with WT mice (P < 0.01) (Fig. 3A and B). Total glycogen phosphorylase activity was also modestly decreased in tibialis anterior muscle (130 ± 2.7 nmol/mg/min in WT versus 119 ± 1.5 nmol/mg/min in muscle-G4KO; P < 0.05) and tended to decrease in gastrocnemius muscle (126 ± 2.5 nmol/mg/min in WT versus 120 ± 1.7 nmol/mg/min in muscle-G4KO; P < 0.08; n = 9) of muscle-G4KO mice compared with that of WT mice.
FIG. 2.
Basal glycogen synthase activity in tibialis anterior (A) and gastrocnemius (B) muscle, and glycogen synthase phosphorylation level (C) and protein amount (D) in gastrocnemius muscle from WT and muscle-G4KO mice. Mice were fasted overnight. (A and B) The ratio of glycogen synthase activity represents the activity measured in the absence divided by that in the presence of glucose-6-phosphate. (C) Proteins in muscle lysates (5 μg) were separated by SDS-PAGE on 8% gels and transferred to nitrocellulose membranes. (D) Proteins in muscle lysates (50 μg) were separated by SDS-PAGE on 8% gels and transferred to nitrocellulose membranes. Glycogen synthase (GS) was visualized by immunoblotting with a glycogen synthase antibody or a phospho-specific glycogen synthase antibody. Results are means ± SEM for five to eight mice per group. OD, optical density. *, P < 0.05 versus WT.
FIG. 3.
Basal glycogen phosphorylase (GP) activity in tibialis anterior (A) and gastrocnemius muscle (B) and contraction-induced glycogen content (C) in soleus muscle from WT and muscle-G4KO mice. Mice were fasted overnight. (A and B) The ratio of glycogen phosphorylase activity represents the activity measured in the absence divided by that in the presence of AMP. (C) After an overnight fast, soleus muscle was dissected. Soleus muscle was electrically stimulated for 10 min (−, noncontraction; +, contraction). The glycogen content was determined by use of a glucose oxidase method. Results are means ± SEM for four to nine mice per group. #, P < 0.05 versus basal (noncontraction) WT; *, P < 0.05 versus basal WT or basal KO.
To understand the mechanism for increased glycogen content, we determined whether glycogen breakdown was impaired in muscle-G4KO mice by measuring the effectiveness of contraction in reducing the glycogen content in soleus muscle. Glycogen content in the basal state was increased by 83% in soleus muscle of muscle-G4KO mice compared with that of WT mice (P < 0.01). Interestingly, in spite of modestly reduced glycogen phosphorylase activity (Fig. 3A and B), contraction decreased glycogen content by ∼40% in soleus muscle of muscle-G4KO, a decrease which was similar to that for WT mice (Fig. 3C).
PI3K, Akt, and GSK3 activities.
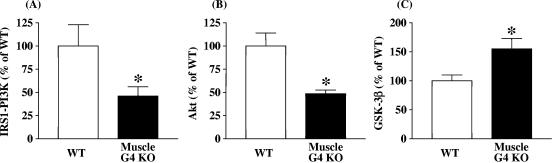
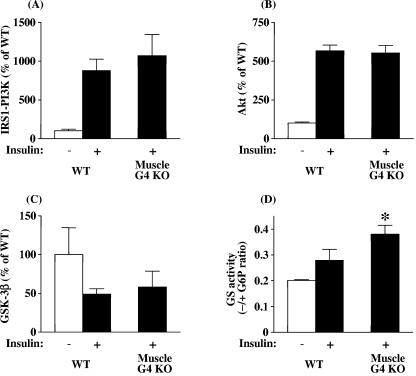
Fasting plasma insulin concentrations are not increased in muscle-G4KO mice (65). Therefore, to determine whether the PI3K-Akt-GSK3 pathway could be upregulated, which could explain the increased basal glycogen synthase activity, we investigated these signaling proteins in muscle. Basal PI3K activity associated with IRS-1 was reduced by 53% in muscle of muscle-G4KO compared with that in WT mice (P < 0.05) (Fig. 4A). Basal Akt activity was also decreased by 52% in muscle of muscle-G4KO compared with that in WT mice (P < 0.05) (Fig. 4B). Correspondingly, GSK3β activity was increased by 55% in muscle-G4KO compared with WT mice (P < 0.05) (Fig. 4C). Levels of basal Akt- and IRS-1-associated PI3K activities were unaltered in adipose tissue from muscle-G4KO mice compared with that of WT mice (data not shown). These results indicate that the increased glycogen content in the skeletal muscle of muscle-G4KO mice cannot be explained by increases in the activity of classical insulin-signaling pathway, since PI3K and Akt activities are decreased, resulting in increased GSK3β activity. This suggests that other pathways are involved in the modulation of glycogen synthase in this insulin-resistant state.
FIG. 4.
Basal IRS-1-associated PI3K (A), Akt (B), and GSK3β (C) activity levels in muscle from WT and muscle-G4KO mice. Mice were fasted overnight. (A) PI3K activities were measured in IRS-1 immunoprecipitates and were quantitated using a PhosphorImager. (B) Muscle lysates (500 μg) were subjected to immunoprecipitation with an Akt antibody that recognizes both Akt1 and Akt2. The immune pellets were assayed for kinase activity using Crosstide as the substrate. (C) Muscle lysates (500 μg) were subjected to immunoprecipitation with a GSK3β-specific antibody. The immune pellets were assayed for kinase activity using phospho-glycogen synthase 1 as the substrate. Results are means ± SEM for four to six mice per group. *, P < 0.05 versus WT.
When mice were acutely stimulated with a large bolus of insulin intravenously (i.v.) to achieve maximal insulin stimulation, IRS-1-associated PI3K and Akt activities were unaltered in muscle-G4KO compared with WT mice (Fig. 5A and B). Insulin inhibited GSK3 activity by ∼50% in muscle of WT and muscle-G4KO mice (Fig. 5C). However, the insulin-stimulated increase in glycogen synthase activity was 36% higher in muscle of muscle-G4KO mice than in that of WT mice (P < 0.05) (Fig. 5D). Even though basal glycogen synthase activity is increased in muscle of muscle-G4KO mice (Fig. 2A and B), insulin stimulates glycogen synthase activity levels 30% higher than basal levels in these mice (compare with Fig. 5D). These data suggest that the deletion of the Glut4 glucose transporter results in increased activity of both basal and insulin-stimulated glycogen synthase in muscle-G4KO compared to WT mice. Insulin-induced insulin receptor and MAPK phosphorylation were unaltered in muscle of muscle-G4KO mice compared with that of WT mice (data not shown). In parallel with the response to an i.v. insulin bolus, prolonged insulin infusion during a euglycemic clamp (2.5 mU insulin/kg/min) (28) also resulted in the normal stimulation of IRS-1-associated PI3K and GSK3, and glycogen synthase activity was also increased by 45% in muscle of muscle-G4KO mice compared with that of WT mice (data not shown).
FIG. 5.
Insulin-stimulated IRS-1-associated PI3K (A), Akt (B), GSK3β (C), and glycogen synthase (D) activity levels in muscle from WT and muscle-G4KO mice. After an overnight fast, mice were injected i.v. with saline (−, white bars) or 10 U/kg insulin (+, black bars). Three min later, muscle was removed. (A) PI3K activities were measured in IRS-1 immunoprecipitates and were quantitated using a PhosphorImager. (B) Muscle lysates (500 μg) were subjected to immunoprecipitation with an Akt antibody that recognizes both Akt1 and Akt2. The immune pellets were assayed for kinase activity using Crosstide as the substrate. (C) Muscle lysates (500 μg) were subjected to immunoprecipitation with a GSK3β-specific antibody. The immune pellets were assayed for kinase activity using phospho-glycan synthase 1 as the substrate. (D) The ratio of glycogen synthase (GS) activity represents the activity measured in the absence divided by that in the presence of glucose-6-phosphate. Results are means ± SEM for four to six mice per group. *, P < 0.05 versus insulin-stimulated WT.
Glycogen-targeting subunit level and activity of PP1.
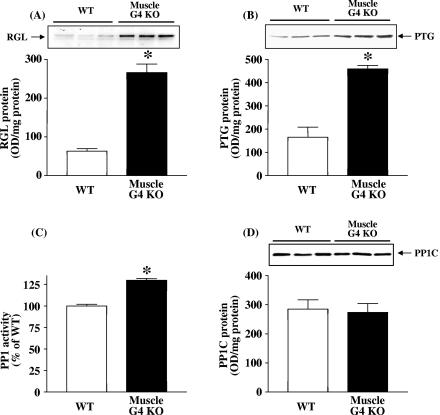
Glycogen-targeting subunits of PP1 play a key role in the regulation of glycogen metabolism (9). PP1 is a regulator of the activation of glycogen synthase and the inactivation of glycogen phosphorylase (9). To determine whether increased expression of glycogen-targeting or regulatory subunits of PP1 could contribute to increased glycogen synthase activity, we measured the protein levels of the regulatory subunits of RGL, PP1G and PTG, in skeletal muscle. Strikingly, the RGL protein level was increased by 4.2-fold and the PTG protein level was increased by 3.4-fold in muscle of muscle-G4KO mice compared with that of WT mice (P < 0.01) (Fig. 6A and B). Both RGL (104% ± 7.8% of WT in muscle-G4KO) and PTG (103% ± 11.9% of WT in muscle-G4KO; P < 0.05) protein levels in the fed state were unaltered in muscle of muscle-G4KO mice compared with that of WT mice.
FIG. 6.
RGL and PTG levels and basal PP1 activity levels and protein amounts in muscle from WT and muscle-G4KO mice. (A and B) Proteins in muscle lysates (25 μg) were separated by SDS-PAGE on 6% or 10% gels and transferred to nitrocellulose membranes. RGL/GM and PTG were visualized by immunoblotting with an RGL or PTG antibody. (C) PP1 activity in muscle homogenates (10 μg) was measured using 32P-labeled phosphorylase a as the substrate. (D) Proteins in muscle lysates (50 μg) were separated by SDS-PAGE on 10% gels and transferred to nitrocellulose membrane. PP1 was visualized by immunoblotting with a PP1 antibody. Results are means ± SEM for 5 to 10 mice per group. OD, optical density. *, P < 0.05 versus WT.
To determine whether the increased levels of RGL and PTG resulted in an increase in PP1 catalytic activity or subunit protein in muscle of muscle-G4KO mice, we measured the PP1 activity and protein levels of the PP1 catalytic subunit. Figure 6C shows that basal PP1 activity was increased by 30% in skeletal muscle of muscle-G4KO mice compared with that of WT mice (P < 0.01). Figure 6D shows a representative blot indicating that the amounts of PP1 catalytic subunit protein were similar in muscle of WT and of muscle-G4KO mice.
Hexokinase II protein and glucose-6-phosphate level.
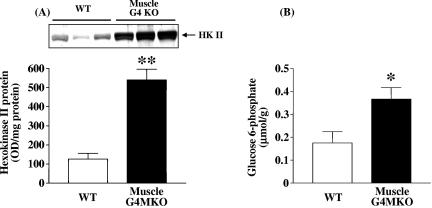
Because glycogen synthase activity is regulated by glucose-6-phosphate levels and glucose-6-phosphate levels are regulated by hexokinase, we measured hexokinase II protein and glucose-6-phosphate levels in muscle. Interestingly, the amount of hexokinase II protein was markedly increased by 4.4-fold in muscle of muscle-G4KO mice compared with that in WT mice (Fig. 7A). In parallel, the glucose-6-phosphate concentration in muscle was also significantly elevated in muscle-G4KO mice compared with that in WT mice (Fig. 7B). These data suggest that increased glucose-6-phosphate levels, possibly resulting from increased hexokinase II activity, may promote an allosteric increase in glycogen synthase activity in these animals, resulting in increased glycogen synthesis.
FIG. 7.
Hexokinase II protein amounts and glucose-6-phosphate concentrations in muscle from WT and muscle-G4KO mice. Mice were fasted overnight. (A) Proteins in muscle lysates (50 μg) were separated by SDS-PAGE on 8% gels and transferred to nitrocellulose membranes. Hexokinase II was visualized by immunoblotting with a hexokinase II antibody. (B) Glucose-6-phosphate concentrations were determined in perchloric acid extracts of frozen muscle tissues. Results are means ± SEM for 6 to 10 mice per group. OD, optical density. *, P < 0.05 versus WT. **, P < 0.01 versus WT.
Glycogen content, glycogen phosphorylase activity, and PTG protein levels in liver.
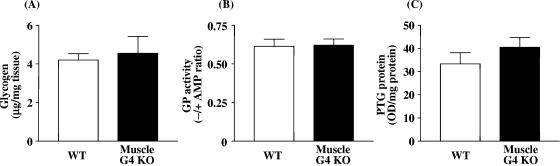
To determine whether the alterations in glycogen metabolism that we see in muscle-G4KO mice are specifically due to the deletion of Glut4 selectively in muscle or due to the altered metabolic state, we examined glycogen content, glycogen phosphorylase activity, and PTG protein levels in liver. Glycogen content and glycogen phosphorylase activity levels were unaltered in liver of muscle-G4KO mice compared with that of WT mice (Fig. 8A and B). PTG protein levels in liver also did not differ significantly between WT mice and muscle-G4KO mice (Fig. 8C). These results indicate that the selective deletion of Glut4 in muscle has no effect on glycogen metabolism in liver in the fasted state. This contrasts with the rapid glycogen synthesis in liver of fasted muscle-G4KO mice following a glucose injection; a glucose injection also stimulates insulin secretion, so the metabolic condition is very different (65). Our results indicate that the altered glycogen metabolism in muscle is most likely directly due to the diminished glucose transport in muscle.
FIG. 8.
Glycogen content levels (A), glycogen phosphorylase activity levels (B), and PTG protein amount (C) in liver from WT and muscle-G4KO mice. Mice were fasted overnight. (A) Liver was solubilized with 0.5 N KOH. The glycogen content was determined by use of a glucose oxidase method. (B) The ratio of glycogen phosphorylase activity represents the activity measured in the absence divided by that measured in the presence of AMP. (C) Proteins in liver lysates (25 μg) were separated by SDS-PAGE on 10% gels and transferred to nitrocellulose membranes. PTG were visualized by immunoblotting with a PTG antibody. Results are means ± SEM for five to eight mice per group. OD, optical density.
Other signaling protein levels.
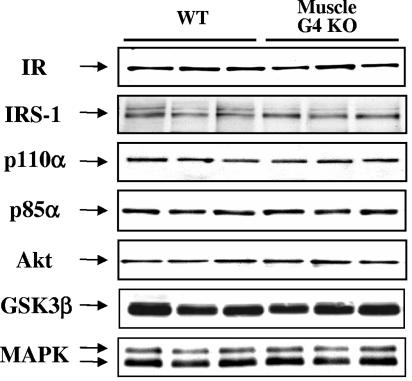
To determine whether changes in the activities of molecules in the signaling cascade are due to alterations in the expression levels of these proteins subsequent to the deletion of Glut4, we measured protein levels for signaling molecules in skeletal muscle of muscle-G4KO mice. The amounts of insulin receptor and IRS-1 protein was not significantly altered in muscle of muscle-G4KO compared with that of WT mice (Fig. 9). Also, the amounts of the p110α catalytic subunit and the p85α regulatory subunit of PI3K and of Akt and GSK3β, respectively, were unaltered in muscle of muscle-G4KO compared with that of WT mice (Fig. 9). The total amount of MAPK protein was not significantly altered in muscle of muscle-G4KO mice (Fig. 9).
FIG. 9.
Signaling protein levels in muscle from WT and muscle-G4KO mice. Mice were fasted overnight. Proteins in muscle lysates (25 to 50 μg) were separated by SDS-PAGE on 6, 8, or 10% gels and transferred to nitrocellulose membranes. Insulin receptor (IR), IRS-1, p110α, p85α, Akt, GSK3α/β, and MAPK were visualized by immunoblotting with specific antibodies. This blot is representative of three blots on six to nine mice per group.
DISCUSSION
Glycogen storage in skeletal muscle is important for both energy metabolism and glucose homeostasis (63). Glucose transport is the rate-limiting step for glycogen synthesis under normal conditions and in type 2 diabetes (11). Therefore, large impairments in glucose transport would be expected to result in decreased glycogen content. Unexpectedly, we found that the deletion of Glut4 selectively in skeletal muscle results in increased glycogen content in both oxidative and glycolytic muscles, despite the fact that glucose transport in these muscles is severely reduced when measured either ex vivo (65) (Fig. 1) or in vivo (28). This raises important questions about the regulation of muscle glycogen content. Interestingly, in mice lacking Glut4 in all tissues, glycogen content and synthesis rates were decreased in some muscles and not in others (53). This may reflect differences in the metabolic milieus of these two models.
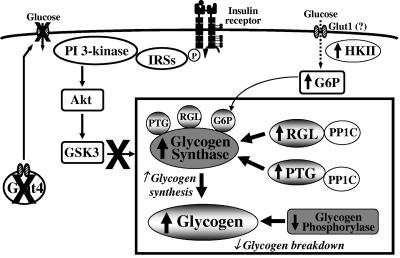
The present study was designed to determine the underlying mechanism(s) for increased glycogen content in muscle of muscle-G4KO mice (Fig. 10). Since glycogen content is controlled predominantly by the coordinated regulation of the two enzymes responsible for its synthesis and breakdown, i.e., glycogen synthase and phosphorylase, respectively (32, 44), we investigated the activities of these two enzymes in muscle of muscle-G4KO mice. A major finding of this study is that glycogen synthase activity in the basal state is increased and glycogen phosphorylase activity is modestly decreased in the absence of Glut4 in muscle. Interestingly, the level of contraction-induced glycogen breakdown is normal despite modestly decreased glycogen phosphorylase activity. These data suggest that increased glycogen content in muscle could be due to increased glycogen synthase activation resulting in enhanced glycogen synthesis.
FIG. 10.
A model of the mechanism(s) by which the muscle-specific deletion of Glut4 results in increased glycogen accumulation in skeletal muscle. Pathways result in both increased glycogen synthesis and decreased glycogen breakdown.
The mechanism for this increased glycogen synthase activity is not increased signaling through the classical pathway regulating glycogen synthesis, since basal PI3K and Akt activities are reduced and GSK3 activity is increased. Instead, there are dramatic changes in several pathways involved in glycogen metabolism. One major alteration is that the abundance of glycogen-targeting subunits of PP1 (RGL and PTG) is increased in skeletal muscle of muscle-G4KO mice. In parallel, PP1 activity is elevated in muscle of muscle-G4KO mice. The activation of PP1 can dephosphorylate and activate glycogen synthase and inactivate glycogen phosphorylase, resulting in increased glycogen accumulation in muscle of muscle-G4KO mice. These data suggest that the abundance of glycogen-targeting subunits can be regulated either directly or indirectly by glucose transport in muscle and that the altered abundance of glycogen-targeting subunits can play an important role in the regulation of glycogen metabolism in muscle in vivo.
We propose that the elevation of the levels of the glycogen-targeting subunits RGL and PTG plays a major role in the increased glycogen content in skeletal muscle of muscle-G4KO mice by activating PP1, which dephosphorylates and activates glycogen synthase. In support of this is the fact that the interaction of glycogen-targeting subunits with PP1C and glycogen synthase is a major determinant of glycogen synthase activation (16, 17, 38), and the overexpression of RGL or PTG causes increased glycogen content and glycogen synthase activity in a variety of cell lines, including human muscle cells (35), CHO cells (35), and isolated hepatocytes (5, 20). Furthermore, a disruption of the RGL gene in mice results in reductions of both glycogen synthase activity and glycogen accumulation in skeletal muscle (14, 54), and a disruption of the PTG gene results in reductions of both parameters in muscle and liver (12). Therefore, it is likely that an increase in glycogen-targeting subunits in skeletal muscle of muscle-G4KO mice results in increased binding of RGL or PTG to PP1C, causing dephosphorylation and the activation of glycogen synthase and leading to increased glycogen content in these mice. RGL most likely plays a greater role than PTG in muscle, since the RGL-knockout animals exhibited a 90% reduction in muscle glycogen levels, despite the preservation of PTG expression in muscle (14).
Note that the increase of glycogen-targeting subunit protein levels is greater than the increase of the PP1 catalytic activity in muscle of muscle-G4KO mice. This difference is most likely due to the methodology for measuring PP1 activity. The current methodology measures both the free form of PP1 activity and the form of PP1 complexed with RGL or PTG or other glycogen-targeting subunits (38). Possibly, the changes in the PP1 catalytic activity specifically complexed with RGL and PTG would be increased to a greater extent. Importantly, the increase in PP1 activity is sufficient to override the inhibitory input from GSK3 on glycogen synthase, because even though GSK3 activity is increased in muscle of muscle-G4KO mice, the phosphorylation of glycogen synthase on the most important site for GSK3 is decreased.
The underlying mechanisms by which the abundance of glycogen-targeting subunits is upregulated in skeletal muscle of muscle-G4KO mice are not known. However, the effect is relatively specific to muscle, in that no changes in PTG levels are seen in liver of these mice. This suggests that the changes in the abundance of glycogen-targeting subunits are not secondary to systemic factors that would affect both tissues. Little is known about the metabolic factors that regulate these scaffolding proteins. A recent study suggests that insulin does not regulate RGL protein levels in skeletal muscle (31). Another study shows that PTG mRNA expression can be induced by neurotransmitters such as noradrenaline and vasoactive intestinal peptide in primary culture of mouse cortical astrocytes (2). These effects are thought to be mediated by β-adrenergic receptor- and cyclic AMP-dependent pathways (1). Future studies will be needed to clarify which factors regulate the expression of glycogen-targeting subunits in skeletal muscle in states of low levels of glucose flux or in the absence of Glut4 protein in vivo.
Since glycogen synthase activity is also regulated by the allosteric effector glucose-6-phosphate, which is directly generated by hexokinase, we measured hexokinase II and glucose-6-phosphate in muscle of muscle-G4KO mice. Interestingly, we found that the deletion of Glut4 in muscle results in increased levels of both hexokinase II and glucose-6-phosphate in muscle of muscle-G4KO mice compared with that of WT mice. These data suggest that the upregulation of hexokinase II leads to increased G6P levels, which may promote an allosteric increase in glycogen synthase activity in these animals, resulting in increased glycogen synthesis. Therefore, increased glycogen synthase activity in muscle of muscle-G4KO mice may also be explained in part by increased levels of glucose-6-phosphate.
In conclusion, our data suggest that mechanisms by which the deletion of Glut4 selectively in muscle results in increased glycogen content in the fasting state could work through the increased activation of glycogen synthase and decreased glycogen phosphorylase activity (Fig. 10). The signaling pathways for increased glycogen synthase activation, at least at low insulin concentrations, appear to be independent of the classical insulin-signaling pathway (PI3K-Akt-GSK3). One involves increases in RGL and PTG proteins with an associated enhancement of the catalytic activity of PP1 in skeletal muscle. Another involves increased hexokinase II, resulting in increased glucose-6-phosphate levels, which allosterically increase glycogen synthase activity. Thus, our data suggest that the muscle-specific deletion of Glut4 alters multiple steps in glycogen metabolism, leading to increased glycogen content in muscle in the fasting state. Understanding the integrated regulation of these pathways in glycogen metabolism could lead to new therapeutic targets for obesity and type 2 diabetes.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants NIH DK43051 and the Metabolic Physiology Core of DK57521 to B.B.K., NIH DK33201 to C.R.K., and NIH DK068626 to L.J.G. and a research grant from the American Diabetes Association to B.B.K. and 7-02-JF-26 to Y.B.K.
We thank M. F. Hirshman for valuable advice and technical assistance and M. F. White, J. Blenis, H. Eldar-Finkelman, H. Haspel, J. Lawrence, A. DePaoli-Roach, and M. Brady for the IRS-1, MAPK GSK3β, Glut4, glycogen synthase, RGL, and PTG antibodies.
REFERENCES
- 1.Allaman, I., S. Lengacher, P. J. Magistretti, and L. Pellerin. 2003. A2B receptor activation promotes glycogen synthesis in astrocytes through modulation of gene expression. Am. J. Physiol. Cell Physiol. 284:C696-C704. [DOI] [PubMed] [Google Scholar]
- 2.Allaman, I., L. Pellerin, and P. J. Magistretti. 2000. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia 30:382-391. [PubMed] [Google Scholar]
- 3.Aschenbach, W. G., Y. Suzuki, K. Breeden, C. Prats, M. F. Hirshman, S. D. Dufresne, K. Sakamoto, P. G. Vilardo, M. Steele, J. H. Kim, S. L. Jing, L. J. Goodyear, and A. A. DePaoli-Roach. 2001. The muscle-specific protein phosphatase PP1G/R(GL) (G(M)) is essential for activation of glycogen synthase by exercise. J. Biol. Chem. 276:39959-39967. [DOI] [PubMed] [Google Scholar]
- 4.Azpiazu, I., J. Manchester, A. V. Skurat, P. J. Roach, and J. C. Lawrence, Jr. 2000. Control of glycogen synthesis is shared between glucose transport and glycogen synthase in skeletal muscle fibers. Am. J. Physiol. Endocrinol. Metab. 278:E234-E243. [DOI] [PubMed] [Google Scholar]
- 5.Berman, H. K., R. M. O'Doherty, P. Anderson, and C. B. Newgard. 1998. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J. Biol. Chem. 273:26421-26425. [DOI] [PubMed] [Google Scholar]
- 6.Bonadonna, R. C., S. Del Prato, E. Bonora, M. P. Saccomani, G. Gulli, A. Natali, S. Frascerra, N. Pecori, E. Ferrannini, D. Bier, C. Cobelli, and R. A. DeFronzo. 1996. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45:915-925. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna, R. C., S. Del Prato, M. P. Saccomani, E. Bonora, G. Gulli, E. Ferrannini, D. Bier, C. Cobelli, and R. A. DeFronzo. 1993. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J. Clin. Investig. 92:486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady, M. J., J. A. Printen, C. C. Mastick, and A. R. Saltiel. 1997. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J. Biol. Chem. 272:20198-20204. [DOI] [PubMed] [Google Scholar]
- 9.Brady, M. J., and A. R. Saltiel. 2001. The role of protein phosphatase-1 in insulin action. Recent Prog. Horm. Res. 56:157-173. [DOI] [PubMed] [Google Scholar]
- 10.Chan, T. M., and J. H. Exton. 1976. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal. Biochem. 71:96-105. [DOI] [PubMed] [Google Scholar]
- 11.Cline, G. W., K. F. Petersen, M. Krssak, J. Shen, R. S. Hundal, Z. Trajanoski, S. Inzucchi, A. Dresner, D. L. Rothman, and G. I. Shulman. 1999. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341:240-246. [DOI] [PubMed] [Google Scholar]
- 12.Crosson, S. M., A. Khan, J. Printen, J. E. Pessin, and A. R. Saltiel. 2003. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J. Clin. Investig. 111:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo, R. A. 1997. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes. Diabetes Rev. 5:177-269. [Google Scholar]
- 14.Delibegovic, M., C. G. Armstrong, L. Dobbie, P. W. Watt, A. J. Smith, and P. T. Cohen. 2003. Disruption of the striated muscle glycogen targeting subunit PPP1R3A of protein phosphatase 1 leads to increased weight gain, fat deposition, and development of insulin resistance. Diabetes 52:596-604. [DOI] [PubMed] [Google Scholar]
- 15.Derave, W., B. F. Hansen, S. Lund, S. Kristiansen, and E. A. Richter. 2000. Muscle glycogen content affects insulin-stimulated glucose transport and protein kinase B activity. Am. J. Physiol. Endocrinol. Metab. 279:E947-E955. [DOI] [PubMed] [Google Scholar]
- 16.Doherty, M. J., G. Moorhead, N. Morrice, P. Cohen, and P. T. Cohen. 1995. Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 375:294-298. [DOI] [PubMed] [Google Scholar]
- 17.Doherty, M. J., P. R. Young, and P. T. Cohen. 1996. Amino acid sequence of a novel protein phosphatase 1 binding protein (R5) which is related to the liver- and muscle-specific glycogen binding subunits of protein phosphatase 1. FEBS Lett. 399:339-343. [DOI] [PubMed] [Google Scholar]
- 18.Furler, S. M., A. B. Jenkins, L. H. Storlien, and E. W. Kraegen. 1991. In vivo location of the rate-limiting step of hexose uptake in muscle and brain tissue of rats. Am. J. Physiol. 261:E337-E347. [DOI] [PubMed] [Google Scholar]
- 19.Garduno, E., M. Nogues, J. M. Merino, C. Gutierrez-Merino, and F. Henao. 2001. The content of glycogen phosphorylase and glycogen in preparations of sarcoplasmic reticulum-glycogenolytic complex is enhanced in diabetic rat skeletal muscle. Diabetologia 44:1238-1246. [DOI] [PubMed] [Google Scholar]
- 20.Gasa, R., C. Clark, R. Yang, A. A. DePaoli-Roach, and C. B. Newgard. 2002. Reversal of diet-induced glucose intolerance by hepatic expression of a variant glycogen-targeting subunit of protein phosphatase-1. J. Biol. Chem. 277:1524-1530. [DOI] [PubMed] [Google Scholar]
- 21.Gilboe, D. P., K. L. Larson, and F. Q. Nuttall. 1972. Radioactive method for the assay of glycogen phosphorylases. Anal. Biochem. 47:20-27. [DOI] [PubMed] [Google Scholar]
- 22.Hansen, P. A., E. A. Gulve, B. A. Marshall, J. Gao, J. E. Pessin, J. O. Holloszy, and M. Mueckler. 1995. Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J. Biol. Chem. 270:1679-1684. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi, T., M. F. Hirshman, E. J. Kurth, W. W. Winder, and L. J. Goodyear. 1998. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47:1369-1373. [DOI] [PubMed] [Google Scholar]
- 24.Huang, X., M. Hansson, E. Laurila, B. Ahren, and L. Groop. 2003. Fat feeding impairs glycogen synthase activity in mice without effects on its gene expression. Metabolism 52:535-539. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki, T. 2000. Insights into insulin resistance and type 2 diabetes from knockout mouse models. J. Clin. Investig. 106:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahn, B. B. 1998. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 92:593-596. [DOI] [PubMed] [Google Scholar]
- 27.Kelley, D. E., K. V. Williams, and J. C. Price. 1999. Insulin regulation of glucose transport and phosphorylation in skeletal muscle assessed by PET. Am. J. Physiol. 277:E361-E369. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J. K., A. Zisman, J. J. Fillmore, O. D. Peroni, K. Kotani, P. Perret, H. Zong, J. Dong, C. R. Kahn, B. B. Kahn, and G. I. Shulman. 2001. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J. Clin. Investig. 108:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, Y. B., S. E. Nikoulina, T. P. Ciaraldi, R. R. Henry, and B. B. Kahn. 1999. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J. Clin. Investig. 104:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, Y. B., G. I. Shulman, and B. B. Kahn. 2002. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda/zeta but not on glycogen synthase kinase-3. J. Biol. Chem. 277:32915-32922. [DOI] [PubMed] [Google Scholar]
- 31.Lanner, C., Y. Suzuki, C. Bi, H. Zhang, L. D. Cooper, M. M. Bowker-Kinley, and A. A. DePaoli-Roach. 2001. Gene structure and expression of the targeting subunit, RGL, of the muscle-specific glycogen-associated type 1 protein phosphatase, PP1G. Arch. Biochem. Biophys. 388:135-145. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, J. C., Jr., and P. J. Roach. 1997. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes 46:541-547. [DOI] [PubMed] [Google Scholar]
- 33.Lerin, C., E. Montell, H. K. Berman, C. B. Newgard, and A. M. Gomez-Foix. 2000. Overexpression of protein targeting to glycogen in cultured human muscle cells stimulates glycogen synthesis independent of glycogen and glucose 6-phosphate levels. J. Biol. Chem. 275:39991-39995. [DOI] [PubMed] [Google Scholar]
- 34.Lin, Y., M. J. Brady, K. Wolanske, R. Holbert, N. B. Ruderman, and G. C. Yaney. 2002. Alterations of nPKC distribution, but normal Akt/PKB activation in denervated rat soleus muscle. Am. J. Physiol. Endocrinol. Metab. 283:E318-E325. [DOI] [PubMed] [Google Scholar]
- 35.Liu, J., and D. L. Brautigan. 2000. Insulin-stimulated phosphorylation of the protein phosphatase-1 striated muscle glycogen-targeting subunit and activation of glycogen synthase. J. Biol. Chem. 275:15940-15947. [DOI] [PubMed] [Google Scholar]
- 36.Lowry, O. H., and J. V. Passonneau. 1972. A flexible system of enzymatic analysis, p. 151-156. Academic Press, New York, N.Y.
- 37.Manchester, J., A. V. Skurat, P. Roach, S. D. Hauschka, and J. C. Lawrence, Jr. 1996. Increased glycogen accumulation in transgenic mice overexpressing glycogen synthase in skeletal muscle. Proc. Natl. Acad. Sci. USA 93:10707-10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newgard, C. B., M. J. Brady, R. M. O'Doherty, and A. R. Saltiel. 2000. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49:1967-1977. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen, J. N., and E. A. Richter. 2003. Regulation of glycogen synthase in skeletal muscle during exercise. Acta Physiol. Scand. 178:309-319. [DOI] [PubMed] [Google Scholar]
- 40.Pederson, B., H. Chen, J. Schroeder, W. Shou, A. Depaoli-Roach, and P. Roach. 2003. Effect of muscle glycogen synthase (GYS1) knockout on cardiac development and glucose homeostasis. Diabetes 52(Suppl. 1):A301-A302. [Google Scholar]
- 41.Pendergrass, M., J. Koval, C. Vogt, H. Yki-Jarvinen, P. Iozzo, R. Pipek, H. Ardehali, R. Printz, D. Granner, R. A. DeFronzo, and L. J. Mandarino. 1998. Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 47:387-394. [DOI] [PubMed] [Google Scholar]
- 42.Pessin, J. E., and A. R. Saltiel. 2000. Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Investig. 106:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen, H. A., P. T. Fueger, D. P. Bracy, D. H. Wasserman, and A. E. Halseth. 2003. Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am. J. Physiol. Endocrinol. Metab. 284:E541-E548. [DOI] [PubMed] [Google Scholar]
- 44.Petersen, K. F., and G. I. Shulman. 2002. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 90:11G-18G. [DOI] [PubMed] [Google Scholar]
- 45.Ragolia, L., and N. Begum. 1997. The effect of modulating the glycogen-associated regulatory subunit of protein phosphatase-1 on insulin action in rat skeletal muscle cells. Endocrinology 138:2398-2404. [DOI] [PubMed] [Google Scholar]
- 46.Ren, J. M., B. A. Marshall, E. A. Gulve, J. Gao, D. W. Johnson, J. O. Holloszy, and M. Mueckler. 1993. Evidence from transgenic mice that glucose transport is rate-limiting for glycogen deposition and glycolysis in skeletal muscle. J. Biol. Chem. 268:16113-16115. [PubMed] [Google Scholar]
- 47.Roach, R. J., and J. Larner. 1977. Covalent phosphorylation in the regulation of glycogen synthase activity. Mol. Cell. Biochem. 15:179-200. [DOI] [PubMed] [Google Scholar]
- 48.Saltiel, A. R. 2001. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517-529. [DOI] [PubMed] [Google Scholar]
- 49.Shulman, G. I. 2000. Cellular mechanisms of insulin resistance. J. Clin. Investig. 106:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shulman, R. G., G. Bloch, and D. L. Rothman. 1995. In vivo regulation of muscle glycogen synthase and the control of glycogen synthesis. Proc. Natl. Acad. Sci. USA 92:8535-8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sreenan, S., S. Keck, T. Fuller, B. Cockburn, and C. F. Burant. 1999. Effects of troglitazone on substrate storage and utilization in insulin-resistant rats. Am. J. Physiol. 276:E1119-E1129. [DOI] [PubMed] [Google Scholar]
- 52.Stalmans, W., M. Bollen, and L. Mvumbi. 1987. Control of glycogen synthesis in health and disease. Diabetes Metab. Rev. 3:127-161. [DOI] [PubMed] [Google Scholar]
- 53.Stenbit, A. E., R. Burcelin, E. B. Katz, T. S. Tsao, N. Gautier, M. J. Charron, and Y. Le Marchand-Brustel. 1996. Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J. Clin. Investig. 98:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki, Y., C. Lanner, J. H. Kim, P. G. Vilardo, H. Zhang, J. Yang, L. D. Cooper, M. Steele, A. Kennedy, C. B. Bock, A. Scrimgeour, J. C. Lawrence, Jr., and A. A. DePaoli-Roach. 2001. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol. Cell. Biol. 21:2683-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang, P. M., J. A. Bondor, K. M. Swiderek, and A. A. DePaoli-Roach. 1991. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J. Biol. Chem. 266:15782-15789. [PubMed] [Google Scholar]
- 56.Villar-Palasi, C., and R. V. Farese. 1994. Impaired skeletal muscle glycogen synthase activation by insulin in the Goto-Kakizaki (G/K) rat. Diabetologia 37:885-888. [DOI] [PubMed] [Google Scholar]
- 57.Virkamaki, A., K. Ueki, and C. R. Kahn. 1999. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Investig. 103:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, K. V., A. Bertoldo, B. Mattioni, J. C. Price, C. Cobelli, and D. E. Kelley. 2003. Glucose transport and phosphorylation in skeletal muscle in obesity: insight from a muscle-specific positron emission tomography model. J. Clin. Endocrinol. Metab. 88:1271-1279. [DOI] [PubMed] [Google Scholar]
- 59.Williams, K. V., J. C. Price, and D. E. Kelley. 2001. Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes 50:2069-2079. [DOI] [PubMed] [Google Scholar]
- 60.Wojtaszewski, J. F., Y. Higaki, M. F. Hirshman, M. D. Michael, S. D. Dufresne, C. R. Kahn, and L. J. Goodyear. 1999. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J. Clin. Investig. 104:1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wojtaszewski, J. F., S. B. Jorgensen, Y. Hellsten, D. G. Hardie, and E. A. Richter. 2002. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51:284-292. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto-Honda, R., Z. Honda, Y. Kaburagi, K. Ueki, S. Kimura, Y. Akanuma, and T. Kadowaki. 2000. Overexpression of the glycogen targeting (G(M)) subunit of protein phosphatase-1. Biochem. Biophys. Res. Commun. 275:859-864. [DOI] [PubMed] [Google Scholar]
- 63.Yeaman, S. J., J. L. Armstrong, S. M. Bonavaud, D. Poinasamy, L. Pickersgill, and R. Halse. 2001. Regulation of glycogen synthesis in human muscle cells. Biochem. Soc. Trans. 29:537-541. [DOI] [PubMed] [Google Scholar]
- 64.Youn, J. H., J. K. Kim, and G. M. Steil. 1995. Assessment of extracellular glucose distribution and glucose transport activity in conscious rats. Am. J. Physiol. 268:E712-E721. [DOI] [PubMed] [Google Scholar]
- 65.Zisman, A., O. D. Peroni, E. D. Abel, M. D. Michael, F. Mauvais-Jarvis, B. B. Lowell, J. F. Wojtaszewski, M. F. Hirshman, A. Virkamaki, L. J. Goodyear, C. R. Kahn, and B. B. Kahn. 2000. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6:924-928. [DOI] [PubMed] [Google Scholar]