Abstract
Yeast-hypha differentiation is believed to be necessary for the normal progression of Candida albicans infections. The emergence and extension of a germ tube from a parental yeast cell are accompanied by dynamic changes in vacuole size and morphology. Although vacuolar function is required during this process, it is unclear if it is vacuolar expansion or some other vacuolar function that is important. We previously described a C. albicans vps11Δ mutant which lacked a recognizable vacuole compartment and with defects in multiple vacuolar functions. These include sensitivities to stress, reduced proteolytic activities, and severe defects in filamentation. Herein we utilize a partially functional VPS11 allele (vps11hr) to help define which vacuolar functions are required for differentiation and which influence interaction with macrophages. Mutant strains harboring this allele are not osmotically or temperature sensitive and have normal levels of secreted aspartyl protease and carboxypeptidase Y activity but have a fragmented vacuole morphology. Moreover, this mutant is defective in filamentation, suggesting that the major role the vacuole plays in yeast-hypha differentiation may relate directly to its morphology. The results of this study support the hypothesis that vacuole expansion is required during germ tube emergence. Both vps11 mutants were severely attenuated in their ability to kill a macrophage cell line. The viability of the vps11Δ mutant was significantly reduced during macrophage interaction compared to that in the control strains, while the vps11hr mutant was unaffected. This implies some vacuolar functions are required for Candida survival within the macrophage, while additional vacuolar functions are required to inflict injury on the macrophage.
The fungal vacuole is an acidic compartment which contains a variety of hydrolytic enzymes and is an important store for cellular metabolites (3, 14). The functions of the fungal vacuole have been well defined for model fungi such as Saccharomyces cerevisiae, and while not essential for vegetative growth, it is important for adaptation to new environments, survival under stressful conditions, and processes of cellular differentiation such as sporulation (3, 28, 36). However, to date, the role the vacuole plays in pathogenic species has not been assessed in the context of adaptation and differentiation within the host.
The opportunistic fungal pathogen Candida albicans is responsible for causing both superficial mucosal and serious systemic infections, the latter associated with neutropenic patients (1). Several “virulence determinants” have been proposed to contribute toward Candida's ability to cause disease, including the ability to secrete aspartyl proteases (secreted aspartyl proteases [SAPs]) and produce a range of morphologically distinct forms, including yeast, hyphae, and pseudohyphae. Gow and Gooday originally observed that the vacuole undergoes a dynamic expansion during C. albicans germ tube emergence from a parental yeast cell (10, 11). Furthermore, during apical extension of the germ tube, asymmetrical division of the protoplasm yields subapical compartments composed almost entirely of vacuole, whereas the protoplasm migrates at the hyphal tip (4, 10). After a delay, the highly vacuolated compartments regenerate cytoplasm and the vacuoles recede. This pattern of vacuole inheritance also seems to influence cell cycle progression and branching frequency of the hyphae (4, 31). At present, the mechanism by which vacuole expansion is mediated and its importance during host interaction are unknown.
S. cerevisiae Vps11p acts as part of a large protein complex required for membrane fusion events at the vacuole and prevacuole compartments (PVC) (22, 26). We have previously shown that a C. albicans vps11Δ strain is defective in vacuole biogenesis and lacks a recognizable vacuole compartment (19). As expected for a strain deficient in vacuolar function, this strain exhibited sensitivity to osmotic and temperature stress and had reduced vacuolar hydrolase activity (as measured by carboxypeptidase Y [CPY] activity). Furthermore, we found the mutant was severely retarded in yeast-hypha differentiation and the secretion of aspartyl proteases, both of which have been implicated in the virulence of this organism. Here we report the analysis of a mutant with a partially functional vps11 allele, as well as additional phenotypic characterization of the original vps11Δ mutant. Our results support that vacuole expansion is required for yeast-hypha differentiation and efficient macrophage killing. We also demonstrate that while not required for yeast-hypha differentiation, the vacuolar hydrolase and stress resistance functions may aid C. albicans survival within the macrophage.
MATERIALS AND METHODS
Strains.
Strains used as part of this study are described in Table 1 and were derived from BWP17 (ura− his− arg−), kindly provided by A. Mitchell (Columbia University) (33).
TABLE 1.
C. albicans strains used in this study
| Strain | Relevant genotype | Parent | Source or reference |
|---|---|---|---|
| BWP17 | ura3Δ/ura3Δ arg4Δ/arg4Δ his1Δ/his1Δ VPS11/VPS11 | SC5314 | 33 |
| YJB6284 | ura3Δ/ura3Δarg4Δ/arg4Δ:ARG4URA3 his1Δ/his1Δ:HIS1VPS11/VPS11 | BWP17 | 5 |
| GPS1 | VPS11/vps11Δ::ARG4 | BWP17 | 19 |
| GPD1 | vps11Δ::ARG4/vps11Δ::URA3 | GPS1 | 19 |
| GPH1 | his1Δ/his1Δ::HIS1 vps11Δ::ARG4/vps11Δ::URA3 | GPD1 | 19 |
| GPR1 | his1Δ/his1Δ::HIS1::VPS11 vps11Δ::ARG4/vps11Δ::URA3 | GPD1 | 19 |
| GPP1-5 | his1Δ/his1Δ::HIS1::vps11hr vps11Δ::ARG4/vps11::URA3 | GPD1 | This study |
Strain GPP1 was generated by transforming the vps11Δ strain, GPD1, with NruI-linearized pGH11D. Correct integration of the vps11hr allele was confirmed by PCR analysis using primer sets HIS3AMP and 11HISR, and the correct genotype was confirmed using 11SEQF and 11SEQR (Table 2), which span the deleted heptad repeat region of VPS11.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) | Purpose |
|---|---|---|
| 11R5 | TCAGGATCCAGGCAATACATGTGATTCG | Amplification of VPS11 and flanking sequences |
| 11R3 | TCAGGATCCATTGGGAGTCCCCCCACTCC | |
| 11SEQF | CCGTTGCATTCAATTTGGGCC | PCR analysis and sequencing of heptad repeat region of VPS11 |
| 11SEQR | CTTTTCATATTTGGCATTGGG | |
| HIS1268F | CCGCTACTGTCTCTACTTTG | Detection of HIS1 integration |
| URA3-5 | CCTATGAATCCACTATTGAACC | Detection of URA3 integration |
| 11HISR | ACTGGGTGCTAGTTCTTTCC | Detection of VPS11 sequences at HIS1 locus |
| HIS3AMP | GTTGGTGTGGCCCAGAGAC |
Construction of the vps11hr mutant allele.
Nucleotides 1448 to 1621 (encoding amino acids 484 to 541) of the VPS11 open reading frame were excised from plasmid pGH11 (19) with ClaI. The resulting product was then self-ligated to produce pGH11D (vps11hr HIS1). Sequencing of pGH11D with primer 11SEQF confirmed the expected in-frame deletion.
Growth conditions.
Strains were routinely grown in yeast extract-peptone-dextrose (YPD) broth at 30°C, unless otherwise stated. For growth curve determination, overnight cultures were subcultured to 20 ml of fresh YPD medium to an optical density at 600 nm (OD600) of 0.2 and incubated at 30°C with shaking. OD600 was determined from samples taken hourly. The murine macrophage cell line J774A.1 (ATCC TIB-67) was grown as per ATCC's instructions in Dulbecco's modified Eagle's medium, high glucose, 4 mM glutamine, and 10% fetal bovine serum at 37°C under 5% CO2. The same culture conditions were used for incubation with Candida.
Phenotypic assays.
Resistance to temperature stress was determined on YPD agar at 37 and 42°C, and that to osmotic stress was determined on YPD agar plus 2.5 M glycerol or 1.5 M NaCl. SAP secretion was examined on bovine serum albumin (BSA) plus YE agar (9). CPY activity was measured using a colorimetric assay as previously reported (19). Resistance to nitrogen starvation was determined using an assay similar to that of Noda et al. (18). Each strain was grown in yeast-nitrogen base (YNB) broth for 48 h at 30°C. Cells were washed twice in distilled water (dH2O), and 107 cells resuspended in 2 ml of medium lacking nitrogen (SD −N). Samples taken at intervals were plated to YPD agar, and viability was determined as CFU after 2 days at 30°C. Filamentation on M199 and 10% fetal calf serum (FCS) agar was performed as previously described (21). Cells from overnight cultures were also induced to filament in 10% FCS (in dH2O) at 37°C after inoculation at 106 cells ml−1. Chlamydospores were induced on cornstarch Tween agar as previously described (20). Vacuole morphology was visualized using two fluorescent dyes, FM4-64 (32) and 5 (and 6-)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCFDA), as previously reported (19). Sensitivity to H2O2, hygromycin B, sodium orthovanadate, and rapamycin was determined by measuring growth in YPD medium supplemented with the appropriate compound. Approximately 1,000 cells from an overnight culture were inoculated to 200 μl growth medium within the wells of a 96-well plate. After 48 h of incubation at 30°C (250 rpm), growth was measured at OD600 using a plate reader.
Phagocytic assays.
C. albicans strains were incubated with the murine macrophage line J774A.1, as described by Lorenz et al. (17). Briefly, J774.A1 cells were seeded overnight in 12-well plates (2 × 105 per well) on 18-mm coverslips and incubated at 37°C under 5% CO2. C. albicans strains were grown overnight at 30°C, washed and incubated with J774A.1 cells at a multiplicity of infection (MOI) of 2.0 (4 × 105/well) unless otherwise noted.
(i) Macrophage survival.
Candida and J774A.1 cells were incubated for 1, 5, and 24 h and then wells were washed and incubated with 0.2 μM calcein AM (final concentration) (LIVE/DEAD Viability/Cytotoxicity kit; Molecular Probes). C. albicans cells were simultaneously stained with calcofluor (0.225 μM). Coverslips were removed from wells and observed under the fluorescent microscope. Macrophages that fluoresced green were viable. Macrophage survival was quantified by counting at least four fields for each well. Results are presented as average numbers of macrophages observed per field.
(ii) Attachment or ingestion.
In order to distinguish between attachment and ingestion, a double-labeling approach was used. After 1 h, wells were washed and cells fixed with 3.7% formaldehyde for 5 min. Postfixing, Candida cells were labeled with calcofluor, which does not enter the macrophage; thus, only exterior Candida cells were labeled. Coverslips were washed and J774A.1 cells were permeabilized with 0.05% Triton X-100 for 20 min at 25°C. Cells were incubated with 0.1 M glycine for 10 min at 25°C to reduce nonspecific binding prior to incubation with a fluorescein-conjugated anti-Candida polyclonal antibody (1/25 dilution) (Biodesign International). Exterior Candida cells were visualized under a 4′,6′-diamidino-2-phenylindole (DAPI) filter, antibody-labeled cells were visualized under a fluorescein filter, and identical images were captured. Images were merged. In order to quantitate association or ingestion, four fields for each well (three wells per strain) were counted and the number of cells ingested (green cells) versus attached only (orange cells) were calculated versus 100 macrophages. Percent attachment or ingestion corresponds to the number of macrophages per 100 that have 1 or more Candida cells attached or ingested, respectively.
(iii) Candida survival.
Candida survival was assessed using an end point dilution assay as described by Rocha et al. (25). Macrophages were seeded overnight in 96-well plates at 5 × 103 per well. Candida cells (50 μl) were added to the first column of cells (150 μl) and then serially diluted 1:4 for six columns so that the resulting MOIs were between 2 × 10−3 and 1.9 × 10−3. The plates were incubated for 48 h. Controls were wells with Candida but no macrophages. The lowest dilution of the control well where it was possible to discriminate distinct colonies was counted. The same dilution was counted for the well containing Candida plus macrophages. Results are presented as numbers of colonies in the presence of macrophages divided by numbers of colonies in the absence of macrophages × 100. Each experiment was set up in quadruplet, and P values were determined using unpaired Student's t test.
RESULTS
Construction of a partially functional vps11 allele.
During the previous study (19), it was noted that the sequence encoding amino acids 484 to 541 could be conveniently deleted. Moreover, sequence analysis of the VPS11 gene product revealed an overlap between this region and a putative heptad repeat region (residues 530 to 558) (19), potentially important in mediating interactions with membranes or other proteins. We therefore constructed a mutant allele lacking the sequence encoding amino acids 484 to 541. This mutant allele (vps11hr) was then introduced into the vps11Δ strain GPD1, to produce the prototrophic strain GPP1, a strain isogenic to the prototrophic deletion (GPH1) and revertant (GPR1) strains previously reported (19). Each strain was analyzed for phenotypes as previously reported. YJB6284 (VPS11/VPS11), and GPR1 were considered positive controls for each experiment.
vps11Δ and vps11hr mutants are differentially affected in vacuolar functions and SAP secretion.
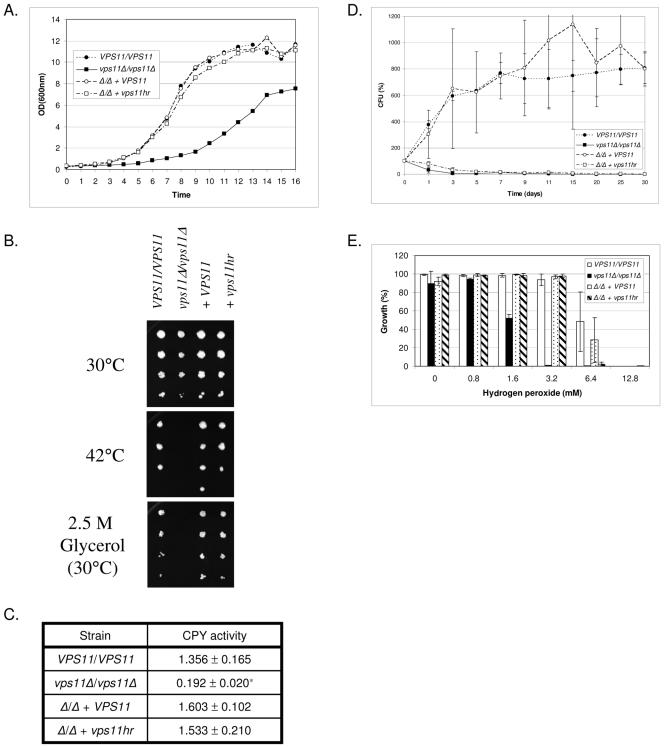
Growth rates were assessed in liquid YPD medium at 30°C. The vps11Δ mutant grew at a significantly slower rate than the VPS11/VPS11 control (Fig. 1A). Reintroduction of either a wild-type copy of VPS11 or the vps11hr allele to the deletion strain restored growth to rates comparable to those with VPS11/VPS11 (Fig. 1A). Resistance to temperature stress was assessed at 42°C. All genotypes grew well at 30 °C (Fig. 1B) and 37°C (data not shown); however, as previously reported (19), the vps11Δ strain was unable to grow at 42°C. The control and vps11hr strains grew comparably well at 42°C (Fig. 1B). A similar pattern of growth was observed under conditions of osmotic stress. On medium containing either 2.5 M glycerol (Fig. 1B) or 1.5 M NaCl (data not shown), vps11Δ was unable to grow, while the control and vps11hr strains grew to similar extents.
FIG. 1.
vps11 mutants are differentially affected in growth and stress resistance. VPS11/VPS11, YJB6284; vps11Δ/vps11Δ, GPH1; vps11Δ/vps11Δ + VPS11, GPR1; vps11Δ/vps11Δ + vps11hr, GPP1. (A) Growth in liquid YPD culture at 30°C was monitored by OD600. Data representative of three experiments are shown. (B) Cell suspensions of each strain were prepared by serial dilution and applied to YPD agar and incubated at 30, 37 (not shown), or 42°C or applied to YPD plus 2.5 M glycerol and incubated at 30°C. (C) CPY activity was measured by colorimetric assay, using a synthetic peptide substrate. Units represent the OD405. Data are the means of three independent experiments. *, P < 0.01. (D) To determine resistance to nitrogen starvation, each strain was incubated in SD −N medium. Samples were taken at the indicated time points, and viability was determined as CFU. Viability is expressed as a percentage of the initial CFU (time zero). Data shown are the means of three independent experiments. (E) Resistance to oxidative stress was determined in liquid YPD medium supplemented with hydrogen peroxide. Growth was measured at OD600 after 48 h at 30°C. Data are the means of three independent experiments.
S. cerevisiae CPY is synthesized as an inactive precursor which is proteolytically activated upon delivery to the vacuole by the action of the vacuolar hydrolase proteinase A (13, 30). Mutants defective in transport steps between the Golgi and PVC or PVC and vacuole mislocalize the CPY precursor and often exhibit reduced CPY activity (2). Thus we measured CPY activity as an indicator of vacuolar hydrolase activity. As previously reported, the vps11Δ strain had significantly reduced levels of CPY activity compared to VPS11+ controls (19). CPY activity in the vps11hr mutant was similar to that in control strains (Fig. 1C).
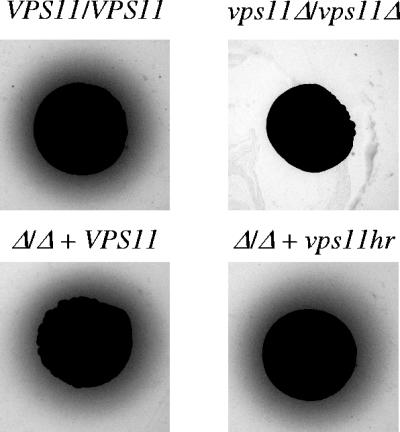
SAP secretion was detected on BSA plus YE agar. Our results confirm the previous finding that the vps11Δ mutant secretes significantly less SAP activity than control strains (19) (Fig. 2). However, the vps11hr mutant had normal SAP activity under these conditions (Fig. 2).
FIG. 2.
vps11 mutants are differentially affected in SAP secretion. SAP activity was detected on BSA plus YE agar. The halo around each colony is caused by SAP-mediated BSA hydrolysis.
vps11 mutants are sensitive to nitrogen starvation.
During periods of nitrogen starvation, portions of cytoplasmic material and entire organelles are delivered to the vacuole via autophagy (28). Degradation of this material is mediated by the vacuolar hydrolases (28), and cellular building blocks such as amino acids are recycled to be used in the synthesis of new proteins. We therefore measured the ability of our mutant strains to resist nitrogen starvation by incubating cells in medium lacking a nitrogen source (SD −N) and measuring viability as CFU from samples taken at time intervals up to 30 days. Upon the shift to SD −N medium, we found that control strains underwent approximately three doublings before growth halted and no significant loss in viability was detected over the duration of the experiment (Fig. 1D). Neither vps11 mutant continued to divide upon shifting to SD −N, and both rapidly lost viability (Fig. 1D).
vps11Δ is sensitive to oxidative stress.
Sensitivity to oxidative stress was determined in YPD medium supplemented with hydrogen peroxide (Fig. 1E). We found the null strain to be approximately fourfold more sensitive to hydrogen peroxide, while the vps11hr strain was only marginally more sensitive than controls (less than twofold).
vps11 mutants are defective in vacuole biogenesis.
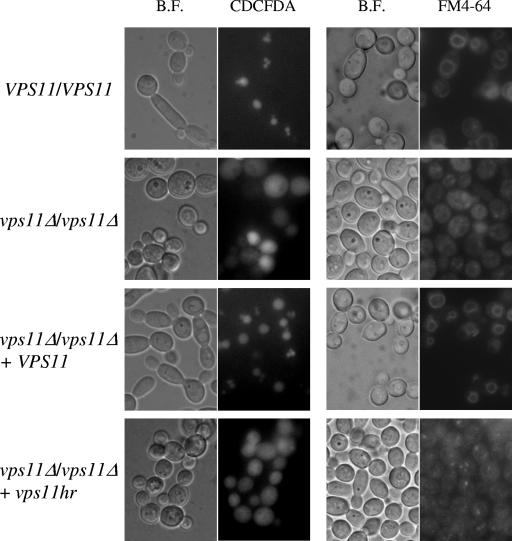
Vacuole morphology was assessed using two fluorescent dyes. FM4-64 is a lipophilic dye which binds to the cellular membrane, is internalized by endocytosis, and accumulates within the vacuole (32). CDCFDA specifically accumulates within the vacuole, where it is hydrolyzed into a fluorescent derivative, and its localization is not dependent upon endocytosis (8). This is important since VPS11 is required for fusion events at the PVC and vacuolar membranes (22, 26) and thus is required for endocytotic trafficking steps. The localization pattern was similar using either dye. Both control strains had one to three large vacuoles per cell (Fig. 3). Both mutant strains had no distinct vacuole compartments and instead had a diffuse staining pattern with CDCFDA or punctate staining with FM4-64. Some intermediate-size compartments were also distinguishable within vps11hr cells (Fig. 3).
FIG. 3.
vps11 mutants have a fragmented-vacuole morphology. (Left panel) Vacuole morphology was visualized using the fluorescent dye CDCFDA. Bright-field (B.F.) and fluorescein isothiocyanate (CDCFDA) images are shown. (Right panel) Vacuole morphology was determined using the lipophilic dye FM4-64.
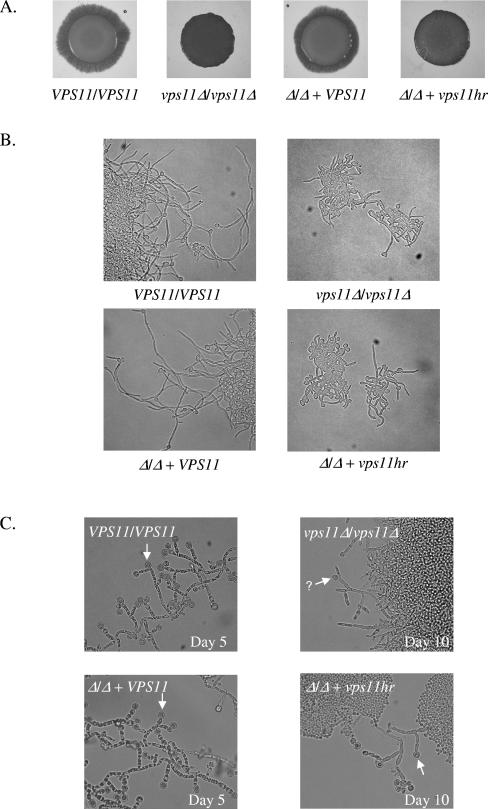
vps11Δ and vps11hr mutants are severely retarded in filamentous growth.
We next examined the mutants' ability to undergo yeast-hypha differentiation. Filamentous growth was induced on solid M199 agar (Fig. 4A) or agar containing fetal calf serum (data not shown). On both media, control strains had produced filaments by day 2, which extended to form a broad filamentous border by day 5. In contrast, neither mutant strain had formed any filaments by day 4. The vps11Δ strain was completely afilamentous under these conditions, while the vps11hr strain eventually produced some filaments by day 5. However, these were much sparser than those for controls, forming short tufts at the colony margin. By day 10, vps11hr filaments remained short and tufty while vps11Δ never filamented. In liquid culture with 10% FCS, the vps11hr strain was defective in filamentation. Germ tube emergence was delayed and the hyphal apex extended less rapidly than for control strains (Fig. 4B). Hyphae of more than two cell compartments in length were never observed, even after extended incubation. The null mutant exhibited severe defects in filamentation with many cells remaining as yeast (Fig. 4B). Those germ tubes that did emerge elongated at a much slower rate and were significantly shorter than the control. Many lacked the parallel cell walls characteristic of true germ tubes. In contrast, control strains rapidly produced parallel-sided germ tubes in liquid FCS medium that extended to form mature hyphae of five to six compartments in length by 5 h (Fig. 4B).
FIG. 4.
vps11 mutants are defective in differentiation. (A) Cell suspensions of each strain were applied as spots to M199 agar and incubated at 37°C. Images were taken at day 4. (B) Each strain was induced to filament in 10% FCS liquid culture at 37°C. Images were taken after 5 h of incubation. (C) Chlamydospore formation was assessed on cornstarch Tween agar, with incubation in the dark. Arrowheads indicate apparently mature chlamydospores, and an arrowhead with a question mark indicates a putatively immature chlamydospore.
VPS11 is required for chlamydospore differentiation.
Chlamydospore formation was observed on cornstarch Tween agar. Control strains formed colonies from which filaments emerged. These filaments gave rise to suspensor cells, which produced abundant chlamydospores by day 5 (Fig. 4C). The vps11Δ mutant formed colonies but almost completely failed to form any filaments or mature chlamydospores. Rounded cell forms that were smaller and less refractile than mature chlamydospores were observed infrequently and only after prolonged incubation (Fig. 4C). These may have been immature chlamydospores. The vps11hr mutant filamented to a similar extent as the vps11Δ strain, producing short filaments sparsely from the colony margins after prolonged incubation. However, these gave rise to apparently normal chlamydospores (Fig. 4C).
vps11 mutants are unable to kill a macrophage cell line.
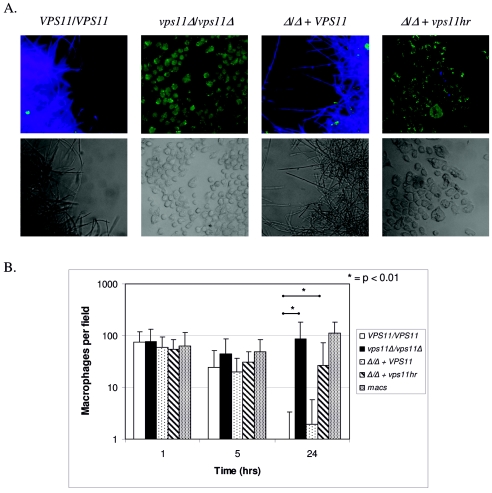
Upon macrophage ingestion, C. albicans cells in the yeast form undergo a rapid switch to the hyphal mode of growth (16, 17, 25). This has been observed to result in the stretching of the macrophage, which may contribute to the observed lysis of the phagocyte. We tested each of our VPS11 strains for its ability to kill the murine macrophage cell line J774A.1. Efficient J774A.1 killing was observed for control strains at the 24-h time point (Fig. 5A and B). However, both vps11 mutant strains were significantly attenuated in their ability to cause death of the J774A.1 cells (Fig. 5A and B). The deletion strain had minimal ability to injure J774A.1 cells, and the number of viable J774A.1 cells at 24 h was not significantly different from that in the control reaction with J774A.1 cells alone. The vps11hr mutant had a somewhat intermediate phenotype, in that some killing of J774A.1 cells was observed. One possibility to account for these results is that the vps11 mutant strains were not as efficiently ingested by the J774A.1 cells as were VPS11+ strains. In order to assess association and ingestion of each strain by J774A.1 cells, we used a dual-staining strategy. Association and ingestion rates were similar for all four strains (Table 3), and thus the differences in macrophage survival are not accounted for by differences in Candida-macrophage interaction.
FIG. 5.
vps11 mutants are deficient in J774A.1 killing. Cultures are as described in Materials and Methods. (A) C. albicans was incubated with J774A.1 cells for 24 h and stained for macrophage viability as described in Materials and Methods. The top panel depicts fluorescent images. Viable macrophages are visualized in green, and C. albicans cells are visualized in blue. Bottom panel shows bright-field images that correspond with the top panel. (B) C. albicans cells were incubated with J774A.1 cells for 1, 5, and 24 h and stained for macrophage viability. The numbers of macrophages were counted per field (four fields per strain). Data are averages of two separate experiments, and Student's unpaired t test was used to determine P values. *, P < 0.01.
TABLE 3.
Association and ingestion of C. albicans strains with J774A.1 cells
| Strain | % Ingestiona | % Associationa |
|---|---|---|
| VPS11/VPS11 | 27.4 ± 13.4 | 7.7 ± 3.7 |
| vps11Δ/vps11Δ | 24.5 ± 9.1 | 14.3 ± 4.2† |
| Δ/Δ + VPS11 | 40.8 ± 7.6 | 5.8 ± 5.1 |
| Δ/Δ + vps11hr | 32.3 ± 7.6 | 7.4 ± 2.4 |
Data represent the number of macrophages with 1 or more associated or ingested Candida out of 100 macrophages. †, P < 0.05 relative to all other strains.
vps11Δ but not vps11hr mutants are susceptible to J774A.1 cells.
To assess if vacuolar function influences Candida survival within the J774A.1 cells, we determined the fate of the ingested mutants. C. albicans survival of the J774A.1 challenge was determined using the end point dilution assay, similar to that described by Whiteway and colleagues (25). Survival of vps11hr cells was not significantly different from that of control strains (Table 4). However, the vps11Δ cells are less able to survive the J774A.1 challenge.
TABLE 4.
C. albicans survival of J774A.1 challenge
| Strain | % Survival at MOI ofa:
|
|
|---|---|---|
| 0.03125:1 | 0.0078:1 | |
| VPS11/VPS11 | 20.3 ± 5.2 | 27.6 ± 11.1 |
| vps11Δ/vps11Δ | 6.9 ± 4.8† | 1.0 ± 2.1‡ |
| Δ/Δ + VPS11 | 18.6 ± 4.0 | 22.9 ± 13.1 |
| Δ/Δ + vps11hr | 15.7 ± 4.0 | 16.0 ± 11.2 |
Candida survival was determined as CFU. Data are given as percent survival relative to the minus macrophage control. †, P < 0.05 relative to all other strains; ‡, P < 0.05 relative to VPS11/VPS11 and Δ/Δ + VPS11 genotypes.
vps11 mutants are sensitive to the drugs hygromycin B, vanadate, and rapamycin.
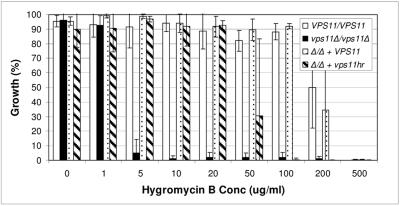
Similar to S. cerevisiae vps11Δ mutants (35), both of our C. albicans vps11 mutants are sensitive to the drug hygromycin B (Fig. 6). The null mutant was approximately 100-fold more sensitive to hygromycin B than either control strain. The vps11hr mutant had an intermediate level of sensitivity: it was approximately 5- to 10-fold more sensitive to hygromycin B than control strains. Similar levels of sensitivity were observed with the drugs rapamycin and sodium orthovanadate (data not shown). This could be suggestive of a role for the vacuole in detoxification of the cytoplasm.
FIG. 6.
vps11 mutants are sensitive to hygromycin B. Growth in YPD medium supplemented with hygromycin B at 30°C was monitored at OD600. Growth is expressed as a percentage of “maximum growth” observed for each strain. Data are expressed as the means of three independent experiments.
DISCUSSION
The vacuole plays a cytologically prominent role during yeast-hypha differentiation of C. albicans. Our previous study demonstrated that vacuolar function(s) or trafficking is required for normal yeast-hypha differentiation (19). However, due to the pleiotropic defects in the vps11Δ mutant, it was unclear which vacuolar function(s) is required for normal filamentation. Herein we report a partially functional vps11 allele (vps11hr), which upon introduction to the vps11Δ strain caused similar defects in yeast-hypha differentiation. However, the vps11hr allele restored normal growth rate, temperature, osmotic, and oxidative stress tolerances, secreted aspartyl protease, and vacuolar CPY activities (Table 5). Thus reconstitution of these vacuolar functions is not sufficient to restore normal filamentation. Instead defects in yeast-hypha differentiation correlate with a fragmented vacuolar morphology and sensitivity to N starvation (Table 5). In S. cerevisiae, APG9 is required for initiation of autophagy in response to nitrogen starvation (18). Preliminary results from a separate project have revealed that a C. albicans mutant with a homologue of APG9 deleted is sensitive to nitrogen starvation but has a “wild-type” vacuole morphology (G. E. Palmer and colleagues, unpublished results). This strain filaments to the same extent as “wild-type” strains under in vitro conditions. Taken together, these studies suggest that the primary role of the vacuole in yeast-hypha differentiation relates to its morphology. This likely reflects the importance of the vacuole expansion observed during germ tube emergence.
TABLE 5.
Phenotypic summary of vps11 mutants
| Parameter | Result for strain:
|
|||
|---|---|---|---|---|
| YJB6284 | GPH1 | GPR1 | GPP1 | |
| Genotype | VPS11/VPS11 | vps11Δ/vps11Δ | vps11Δ/vps11Δ + VPS11 | vps11Δ/vps11Δ + vps11hr |
| Growth at 30°C | +++++ | ++ | +++++ | +++++ |
| Temp stress (42°C) | +++++ | − | +++++ | +++++ |
| Osmotic stress | +++++ | − | +++++ | +++++ |
| CPY activity | +++++ | − | +++++ | +++++ |
| N starvation | +++++ | − | +++++ | + |
| SAP secretion | +++++ | − | +++++ | +++++ |
| Oxidative stress | +++++ | + | +++++ | ++++ |
| Vacuole morphology | +++++ | − | +++++ | + |
| Filamentation | +++++ | − | +++++ | + |
| Chlamydospore differentiation | +++++ | − | +++++ | + |
| Macrophage survival | +++++ | + | +++++ | ++++ |
| Macrophage killing | +++++ | − | ++++ | + |
| Drug resistance | +++++ | − | +++++ | ++ |
To facilitate vacuole expansion during germ tube emergence, new membrane material must be incorporated at the vacuole surface. S. cerevisiae Vps11p localizes to the membrane of the vacuole and prevacuole compartments, where it forms part of a large protein complex (HOPS complex) which mediates the fusion of transport vesicles with these compartments (22, 24, 34). It is therefore likely that the vacuole expansion observed during C. albicans germ tube outgrowth is dependent upon Vps11p-mediated membrane fusion events at the PVC and vacuolar membrane. The source of the membrane incorporated into the vacuole has yet to be determined, as has the regulation of these events. The inability of our vps11 mutants to undergo vacuole expansion limits the rate of apical extension to what can be achieved by cytoplasmic biosynthesis. The inability of the mutants to sustain filamentous growth and form mature filaments also suggests that vacuole expansion is required for the maintenance of polarized growth. This could result from a loss of osmotic pressure in the mutant cells, which has been proposed to help direct apical growth (15).
One potential benefit of vacuole expansion may be to reduce the need for cytoplasmic biosynthesis, while permitting rapid extension of the hypha. This would be particularly important under conditions of nutrient limitation. The model eukaryote S. cerevisiae does not form true hyphae, and while vacuole morphology is regulated in response to growth phase and osmotic conditions (6), there are no comparable vacuole expansion events. However, some plant pathogens, such as Magnaporthe grisea and Ustilago maydis, utilize a similar mechanism of vacuolar expansion during apical extension of hyphae (27). This may permit these pathogens to explore host surfaces where nutrients are limiting.
Both vps11 mutants had severe defects in chlamydospore differentiation on cornstarch agar. Neither strain produced filaments; thus it was unclear if the lack of chlamydospores was merely a secondary consequence of an inability to filament or if these strains are inherently defective in chlamydospore differentiation. Our results suggest that the vps11hr mutant is competent to form chlamydospores, but their abundance is severely limited by the poor filamentation of this strain, while the vps11Δ mutant is not competent to form either filaments or chlamydospores. This could indicate that alternate vacuolar functions are required during chlamydospore formation than during filamentous growth. It is known that sporulation of S. cerevisiae is dependent upon vacuolar hydrolase activity, and it is possible that chlamydospore differentiation in C. albicans has similar requirements.
The lack of SAP activity in the vps11Δ mutant may indicate that SAPs are secreted via the PVC. The restoration of SAP activity in the vps11hr mutant implies that a trafficking step, which SAP depends upon, is restored in this strain. These results suggest that trafficking through the PVC compartment is unaffected in the vps11hr mutant. Thus, the few intermediate-size compartments observed with the FM4-64 dye may represent PVCs. In this case, restoring trafficking through the PVC in the vps11Δ strain is not sufficient to restore filamentous growth and it is trafficking to the vacuole itself which is required.
Both vps11 mutants exhibited sensitivity to the drugs hygromycin B, orthovanadate, and rapamycin. This may be indicative of a role for the vacuole in drug detoxification. One possibility is the drugs are actively removed from the cytoplasm and sequestered within the vacuole. Intriguingly, Theiss et al. (29) have described an ABC-like transporter with similarity to the multidrug resistance transporters, which localizes to the C. albicans vacuole. Both vps11 mutants were also sensitive to nitrogen starvation, which reflects the importance of the vacuole in recycling cellular proteins through autophagy. The sensitivity of both strains is liable to be the result of the absence of a suitable “recipient” compartment for the autophagosome. In the case of the vps11Δ strain, the lack of vacuolar hydrolase activity may also limit the strain's capacity to recycle cellular proteins.
The biological relevance of these results was made apparent in our J774A.1 challenge experiments. Previous reports have suggested two main mechanisms by which Candida might cause macrophage death. First, filamentation within the macrophage causes visible stretching of the macrophage, and this mechanical stress may lead to macrophage lysis (17, 25). Second, an enzyme(s) secreted by C. albicans, such as the SAPs, causes macrophage injury (7). Both vps11Δ and vps11hr mutants were defective in filamentation, but only the vps11Δ mutant had reduced SAP activity. The vps11Δ mutant cells were completely unable to kill the J774A.1 macrophages, whereas the vps11hr mutant killed the macrophage cell line at a level intermediate between those of the vps11Δ and VPS11+ strains. Therefore, SAP activity may contribute to but is not adequate for efficient macrophage killing. The attenuated macrophage killing by the vps11hr mutant is likely due to the poor filamentation of this strain. While both mutants were sensitive to nitrogen starvation, this is less likely to represent a mechanism leading to macrophage death due to the relatively short length of the Candida-macrophage interaction. This is supported by preliminary experiments with the nitrogen starvation-sensitive apg9Δ mutant, which is not deficient in macrophage killing (unpublished results). However, we feel that resistance to nitrogen starvation may be important for the long-term survival of Candida within a host.
While the vps11Δ mutant is killed by J774A.1 cells, the vps11hr strain is not. Therefore vacuolar functions in resistance to stresses and/or vacuolar and secreted protease activities help Candida survive within the macrophage following phagocytosis. The sensitivity of the vps11Δ strain to oxidative stress is liable to render it more sensitive to J774A.1 killing. This sensitivity is probably a secondary consequence of the displacement of transition metals usually stored in the vacuole (23), resulting in increased cytoplasmic concentrations. This would make the cells sensitive to oxidative stress as these metals catalyze the generation of reactive oxygen species (12).
The rapid germination and extension of the C. albicans germ tube seem to depend upon the formation of enlarged vacuole compartments. This capability may be critical in mediating escape from and injury of phagocytes. However, additional vacuolar functions are required for survival within macrophages and may also be necessary for colonization and invasion of the host.
Acknowledgments
This work was supported by NIAID/NIH grants RO1AI46142 and R21AI60371 awarded to J.S.
REFERENCES
- 1.Ashman, R. B., C. S. Farah, S. Wanasaengsakul, Y. Hu, G. Pang, and R. L. Clancy. 2004. Innate versus adaptive immunity in Candida albicans infection. Immunol. Cell Biol. 82:196-204. [DOI] [PubMed] [Google Scholar]
- 2.Bankaitis, V. A., L. M. Johnson, and S. D. Emr. 1986. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. USA 83:9075-9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banta, L. M., J. S. Robinson, D. J. Klionsky, and S. D. Emr. 1988. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107:1369-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barelle, C. J., E. A. Bohula, S. J. Kron, D. Wessels, D. R. Soll, A. Schäfer, A. J. P. Brown, and N. A. Gow. 2003. Asynchronous cell cycle and asymmetric vacuolar inheritance in true hyphae of Candida albicans. Eukaryot. Cell 2:398-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensen, E. S., S. G. Filler, and J. Berman. 2002. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot. Cell 1:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonangelino, C. J., J. J. Nau, J. E. Duex, M. Brinkman, A. E. Wurmser, J. D. Gary, S. D. Emr, and L. S. Weisman. 2002. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 156:1015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard, and M. Monod. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543-554. [DOI] [PubMed] [Google Scholar]
- 8.Conibear, E., and T. H. Stevens. 2002. Studying yeast vacuoles. Methods Enzymol. 351:408-432. [DOI] [PubMed] [Google Scholar]
- 9.Crandall, M., and J. E. Edwards, Jr. 1987. Segregation of proteinase-negative mutants from heterozygous Candida albicans. J. Gen. Microbiol. 133:2817-2824. [DOI] [PubMed] [Google Scholar]
- 10.Gow, N. A., and G. W. Gooday. 1984. A model for the germ tube formation and mycelial growth form of Candida albicans. Sabouraudia 22:137-144. [DOI] [PubMed] [Google Scholar]
- 11.Gow, N. A., and G. W. Gooday. 1982. Vacuolation, branch production and linear growth of germ tubes in Candida albicans. J. Gen. Microbiol. 128:2195-2198. [DOI] [PubMed] [Google Scholar]
- 12.Gutteridge, J. M., and B. Halliwell. 1989. Iron toxicity and oxygen radicals. Baillieres Clin. Haematol. 2:195-256. [DOI] [PubMed] [Google Scholar]
- 13.Hemmings, B. A., G. S. Zubenko, A. Hasilik, and E. W. Jones. 1981. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 78:435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klionsky, D. J., P. K. Herman, and S. D. Emr. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch, A. L. 1994. The problem with hyphal growth in streptomycetes and fungi. J. Theor. Biol. 171:137-150. [Google Scholar]
- 16.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz, M. C., J. A. Bender, and G. R. Fink. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noda, T., J. Kim, W. P. Huang, M. Baba, C. Tokunaga, Y. Ohsumi, and D. J. Klionsky. 2000. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J. Cell Biol. 148:465-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, G. E., A. Cashmore, and J. Sturtevant. 2003. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot. Cell 2:411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer, G. E., K. J. Johnson, S. Ghosh, and J. Sturtevant. 2004. Mutant alleles of the essential 14-3-3 gene in Candida albicans distinguish between growth and filamentation. Microbiology 150:1911-1924. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, G. E., and J. E. Sturtevant. 2004. Random mutagenesis of an essential Candida albicans gene. Curr. Genet. 46:343-356. [DOI] [PubMed] [Google Scholar]
- 22.Peterson, M., and S. Emr. 2001. The class C Vps complex functions at multiple stages of the vacuolar transport pathway. Traffic 2:476-486. [DOI] [PubMed] [Google Scholar]
- 23.Raguzzi, F., E. Lesuisse, and R. R. Crichton. 1988. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 231:253-258. [DOI] [PubMed] [Google Scholar]
- 24.Rieder, S. E., and S. D. Emr. 1997. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol. Biol. Cell 8:2307-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha, C. R., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12:3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava, A., C. A. Woolford, and E. W. Jones. 2000. Pep3p/Pep5p complex: a putative docking factor at multiple steps of vesicular transport to the vacuole of Saccharomyces cerevisiae. Genetics 156:105-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg, G., M. Schliwa, C. Lehmler, M. Bolker, R. Kahmann, and J. R. McIntosh. 1998. Kinesin from the plant pathogenic fungus Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J. Cell Sci. 111:2235-2246. [DOI] [PubMed] [Google Scholar]
- 28.Takeshige, K., M. Baba, S. Tsuboi, T. Noda, and Y. Ohsumi. 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119:301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theiss, S., M. Kretschmar, T. Nichterlein, H. Hof, N. Agabian, J. Hacker, and G. A. Kohler. 2002. Functional analysis of a vacuolar ABC transporter in wild-type Candida albicans reveals its involvement in virulence. Mol. Microbiol. 43:571-584. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Hazel, H. B., M. C. Kielland-Brandt, and J. R. Winther. 1996. Review: biosynthesis and function of yeast vacuolar proteases. Yeast 12:1-16. [DOI] [PubMed] [Google Scholar]
- 31.Veses, V., M. Casanova, Á. Murgui, A. Dominguez, N. A. R. Gow, and J. P. Martinez. 2005. ABG1, a novel and essential Candida albicans gene encoding a vacuolar protein involved in cytokinesis and hyphal branching. Eukaryot. Cell 4:1088-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurmser, A. E., T. K. Sato, and S. D. Emr. 2000. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J. Cell Biol. 151:551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida, S., and Y. Anraku. 2000. Characterization of staurosporine-sensitive mutants of Saccharomyces cerevisiae: vacuolar functions affect staurosporine sensitivity. Mol. Gen. Genet. 263:877-888. [DOI] [PubMed] [Google Scholar]
- 36.Zubenko, G. S., and E. W. Jones. 1981. Protein degradation, meiosis and sporulation in proteinase-deficient mutants of Saccharomyces cerevisiae. Genetics 97:45-64. [DOI] [PMC free article] [PubMed] [Google Scholar]